Section 1 Chemical Changes Essential Questions What are






- Slides: 15

Section 1 Chemical Changes

Essential Questions What are the reactants and products in a chemical reaction? Is mass conserved in a chemical reaction? Why are chemical equations important? How do you balance a chemical equation?

Review Vocabulary chemical formula: shorthand that gives the elements in a compound and their ratios

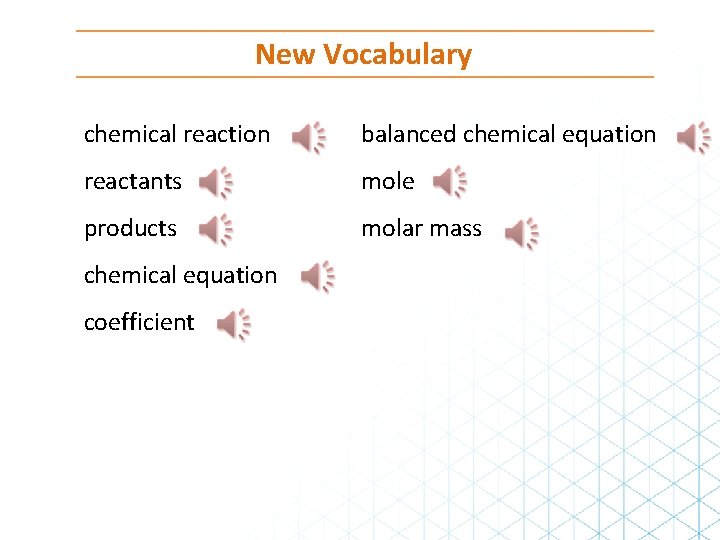
New Vocabulary chemical reaction balanced chemical equation reactants mole products molar mass chemical equation coefficient

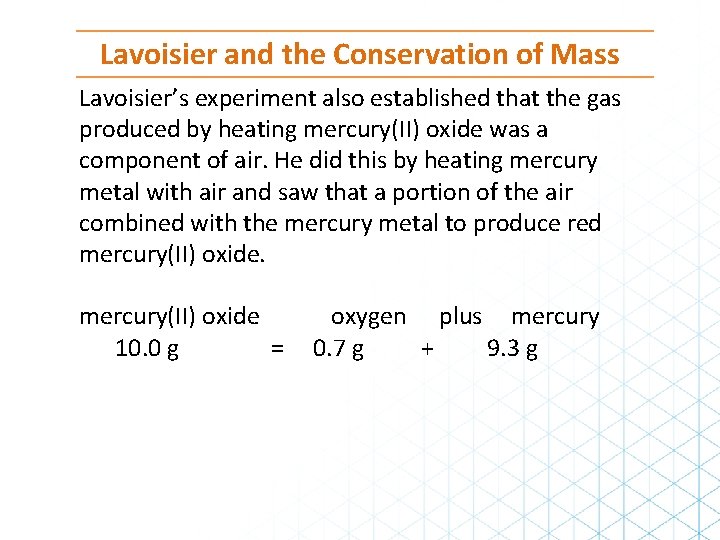
Lavoisier and the Conservation of Mass Lavoisier’s experiment also established that the gas produced by heating mercury(II) oxide was a component of air. He did this by heating mercury metal with air and saw that a portion of the air combined with the mercury metal to produce red mercury(II) oxide 10. 0 g = oxygen plus mercury 0. 7 g + 9. 3 g

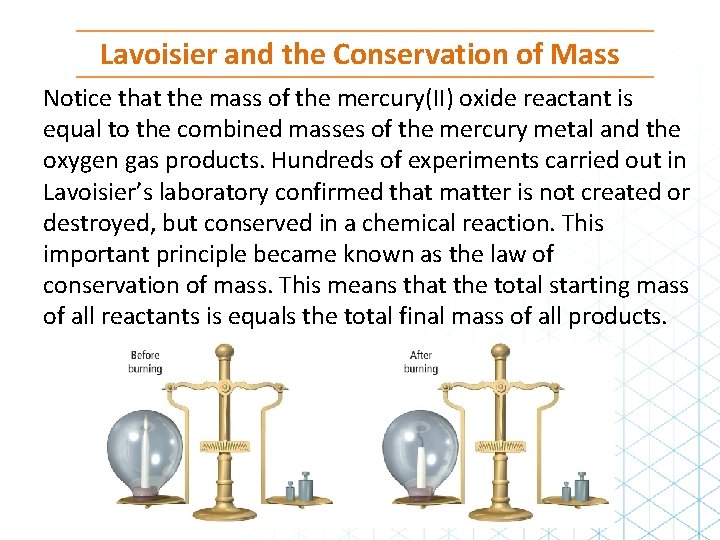
Lavoisier and the Conservation of Mass Notice that the mass of the mercury(II) oxide reactant is equal to the combined masses of the mercury metal and the oxygen gas products. Hundreds of experiments carried out in Lavoisier’s laboratory confirmed that matter is not created or destroyed, but conserved in a chemical reaction. This important principle became known as the law of conservation of mass. This means that the total starting mass of all reactants is equals the total final mass of all products.

Writing Equations Many words are needed to state all the important information about reactions. As a result, scientists developed a shorthand method to describe chemical reactions. A chemical equation is a way to describe a chemical reaction using chemical formulas and other symbols. Some of the symbols used in chemical equations are listed in the table below.

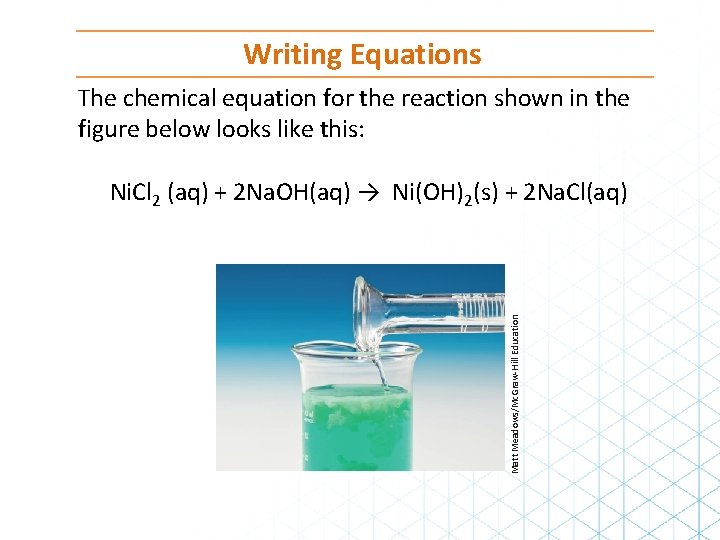
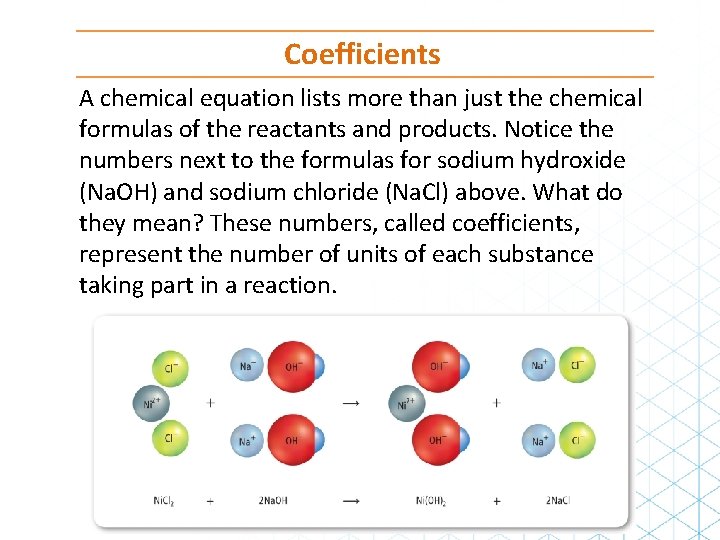
Writing Equations The chemical equation for the reaction shown in the figure below looks like this: Matt Meadows/Mc. Graw-Hill Education Ni. Cl 2 (aq) + 2 Na. OH(aq) → Ni(OH)2(s) + 2 Na. Cl(aq)

Coefficients A chemical equation lists more than just the chemical formulas of the reactants and products. Notice the numbers next to the formulas for sodium hydroxide (Na. OH) and sodium chloride (Na. Cl) above. What do they mean? These numbers, called coefficients, represent the number of units of each substance taking part in a reaction.

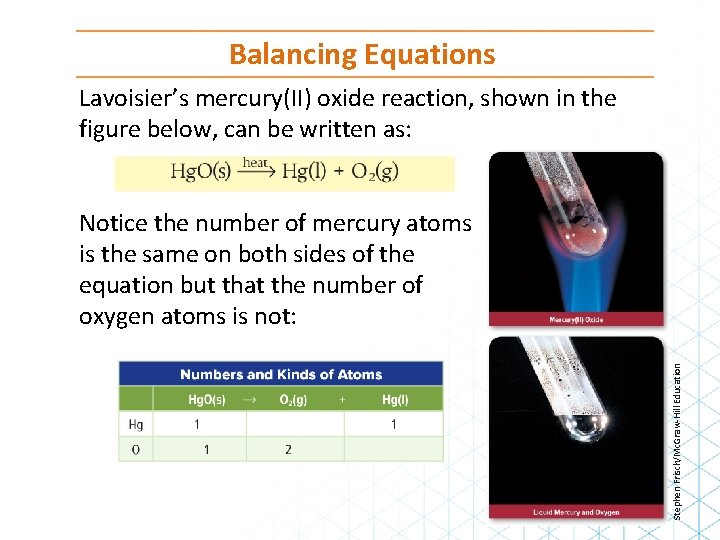
Balancing Equations Lavoisier’s mercury(II) oxide reaction, shown in the figure below, can be written as: Stephen Frisch/Mc. Graw-Hill Education Notice the number of mercury atoms is the same on both sides of the equation but that the number of oxygen atoms is not:

Understanding Chemical Equations AIC/age fotostock When charcoal burns, as shown in the figure below, heat is liberated by the chemical reaction between carbon in the charcoal and oxygen in the air. Molecules of carbon dioxide gas are produced. Note in the balanced equation for this reaction that one oxygen molecule (O 2) is required for each carbon atom and that one carbon dioxide molecule (CO 2) is produced. Also, we know from the periodic table that the average mass of a carbon atom is 12. 01 amu and the average mass of an oxygen atom is 16. 00 amu. Therefore, the average mass of an O 2 molecule is 32. 00 amu (2 × 16. 00 amu) and the average mass of a CO 2 molecule is 44. 01 amu (12. 01 amu + (2 × 16. 00 amu)).

Assessment 1. When hydrogen burns, what is oxygen’s role? A catalyst B inhibitor C product D reactant CORRECT

Assessment 2. Which best describes the reactants and products in a reaction at equilibrium? A They form at equal rates. B More reactants form than products. C More products form than reactants. D They form equal amounts. CORRECT

Assessment 3. How do you indicate that a substance in an equation is a solid? A (l) B (g) C (s) D (aq) CORRECT

Assessment 4. Oxygen gas is always written as O 2 in chemical equations. What term is used to describe the 2 in this formula? A product B coefficient C catalyst D subscript CORRECT