Physical vs Chemical Changes Comparing Changes Chemical Change







- Slides: 13

Physical vs. Chemical Changes

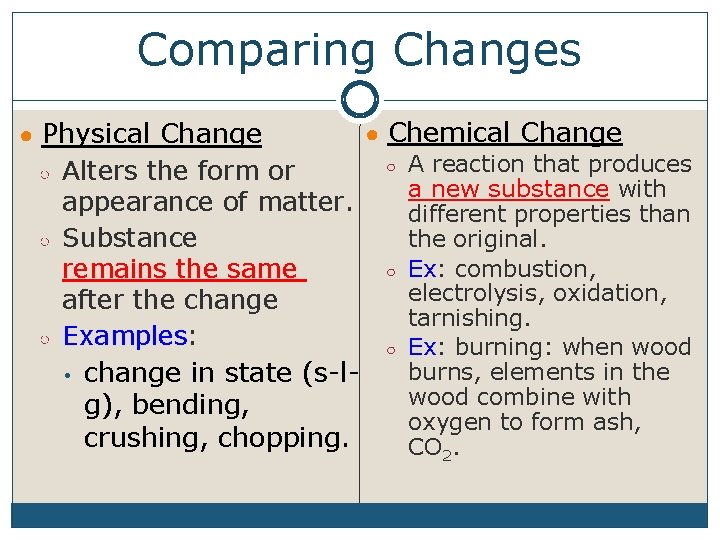
Comparing Changes ● Chemical Change ○ A reaction that produces ○ Alters the form or a new substance with appearance of matter. different properties than ○ Substance the original. ○ Ex: combustion, remains the same electrolysis, oxidation, after the change tarnishing. ○ Examples: ○ Ex: burning: when wood burns, elements in the • change in state (s-lwood combine with g), bending, oxygen to form ash, crushing, chopping. CO 2. ● Physical Change

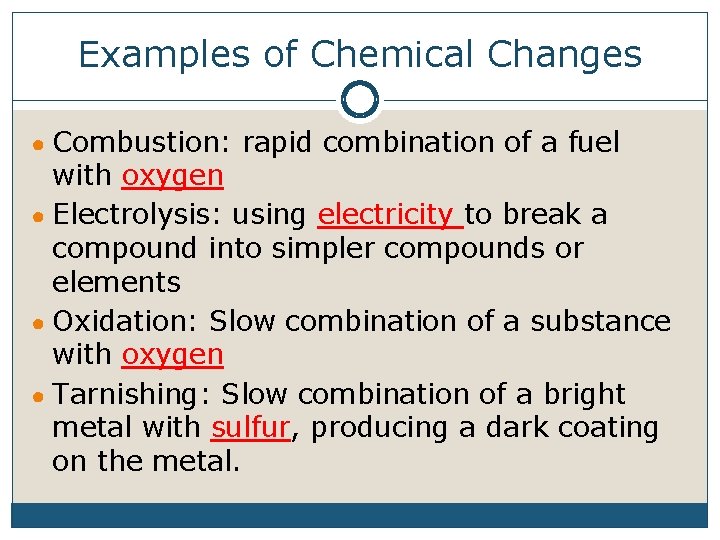
Examples of Chemical Changes ● Combustion: rapid combination of a fuel with oxygen ● Electrolysis: using electricity to break a compound into simpler compounds or elements ● Oxidation: Slow combination of a substance with oxygen ● Tarnishing: Slow combination of a bright metal with sulfur, producing a dark coating on the metal.





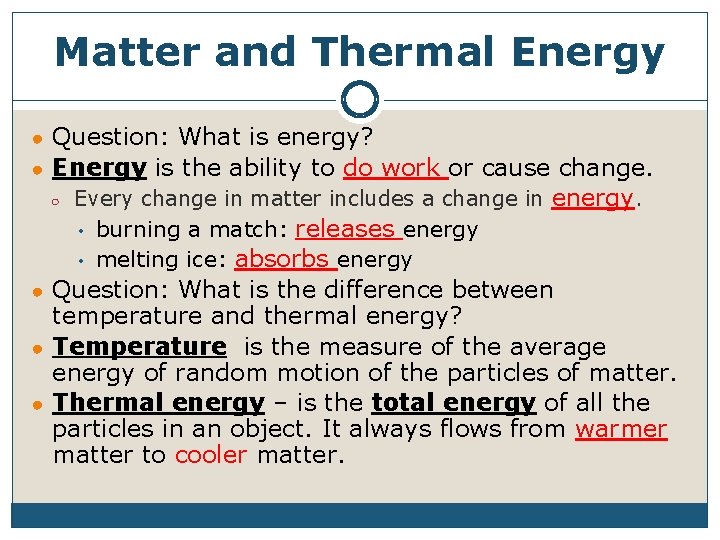
Matter and Thermal Energy ● Question: What is energy? ● Energy is the ability to do work or cause change. ○ Every change in matter includes a change in energy. • • burning a match: releases energy melting ice: absorbs energy ● Question: What is the difference between temperature and thermal energy? ● Temperature is the measure of the average energy of random motion of the particles of matter. ● Thermal energy – is the total energy of all the particles in an object. It always flows from warmer matter to cooler matter.

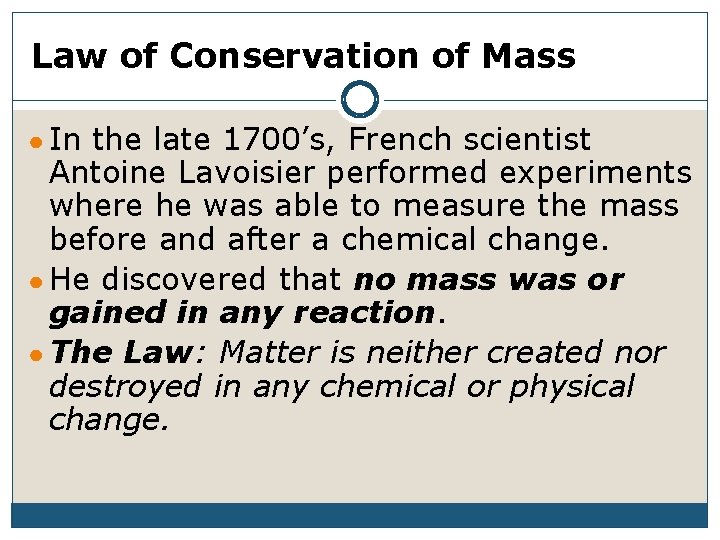
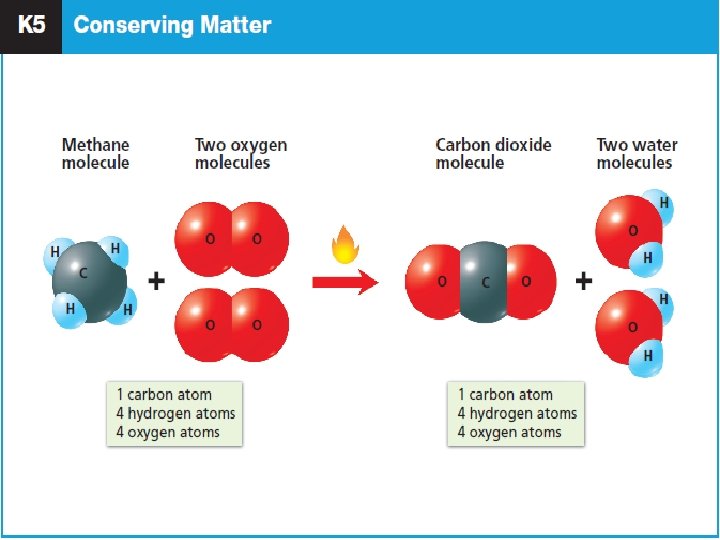
Law of Conservation of Mass ● In the late 1700’s, French scientist Antoine Lavoisier performed experiments where he was able to measure the mass before and after a chemical change. ● He discovered that no mass was or gained in any reaction. ● The Law: Matter is neither created nor destroyed in any chemical or physical change.

Thermal Energy vs. Temp ● Question 1: If you remove a cup of water from the ocean, does the water have the same temperature as the ocean? Answer: Yes! ● Question 2: Do both have the same thermal energy? Answer: No. The ocean has much more thermal energy than the cup of ocean water because the ocean has much more mass.

An Ice Cube? ● So, looking at the following two scenarios, describe the transfer of thermal energy. Which way is thermal energy flowing? : ○ 1. An ice cube melting on a counter top Thermal energy flows from the counter top and surrounding air (warmer) to the ice cube (cooler), causing it to melt. ○ 2. Ice cubes that are placed into a room-temperature soda Thermal energy flows from the soda (warmer) to the ice cubes (cooler), causing the ice to melt and the soda to get cooler. So technically, the ice cubes don’t transfer “cold” to the soda, but instead the soda “heats” the ice.

Endothermic vs. Exothermic Change ● When matter changes, it can either be: ● Endothermic: absorbing energy ○ Examples: an ice cube melting, photosynthesis ● Exothermic: releasing energy ○ Examples: a burning match, a fire, the sun
