Chemical Changes in Matter Physical Vs Chemical Changes














- Slides: 16

Chemical Changes in Matter

Physical Vs. Chemical Changes • Physical Change: is a change in the characteristics of a substance. • Chemical Change: is a change from one substance into another substance.

• One of the most important chemical changes on Earth is photosynthesis. * green plants use sunlight, carbon dioxide and water to make sugar and oxygen.

• Another important chemical change takes place in the cells in your body during respiration. * the cells combine sugar and oxygen to produce energy, carbon dioxide and water

Chemical Reaction • A WELL defined example of a chemical change. * one or more substances are changed to new substances

Reactants • The substances that are about to change. * the stuff going into the reaction * found on the left side

Products • The new substances created by the reaction. * the stuff you get after the reaction * found on the right side

Reactants Products • Shows the relationship between both sides. • The arrow is read as produces or yields.

Law of Conservation of Mass • In a chemical reaction, matter is NOT created or destroyed only converted (changed). * you get what you pay for

• When describing a chemical reaction, the Law of Conservation of Mass MUST be satisfied.

• Chemical reactions need lots of words to state all the important information. * science developed a shorthand method to describe a chemical reaction

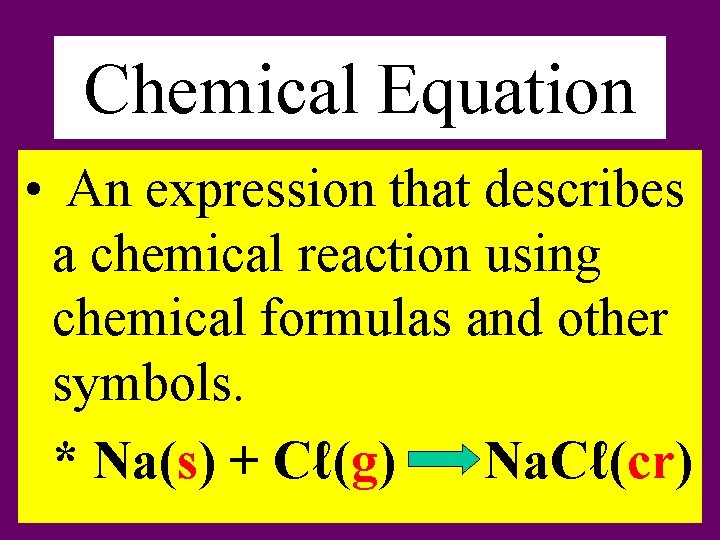
Chemical Equation • An expression that describes a chemical reaction using chemical formulas and other symbols. * Na(s) + Cℓ(g) Na. Cℓ(cr)

• What does the symbols (cr), (g), (aq), (ℓ) and (s) mean in the equations? * (cr) = crystalline solid * (g) = gas * (aq) = aqueous [dissolved in water] * (ℓ) = liquid * (s) = solid

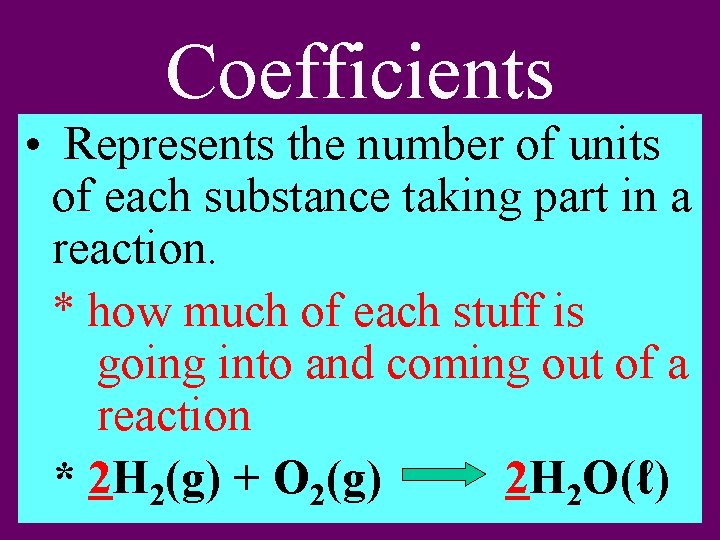
Coefficients • Represents the number of units of each substance taking part in a reaction. * how much of each stuff is going into and coming out of a reaction * 2 H 2(g) + O 2(g) 2 H 2 O(ℓ)

Coefficients • If no number is given we assume the number one (1). * 2 H 2(g) + 1 O 2(g) 2 H 2 O(ℓ)
