Ruther ford experiment Video 1 Video 2 Video





- Slides: 14

Ruther ford experiment Video 1 Video 2 Video 3

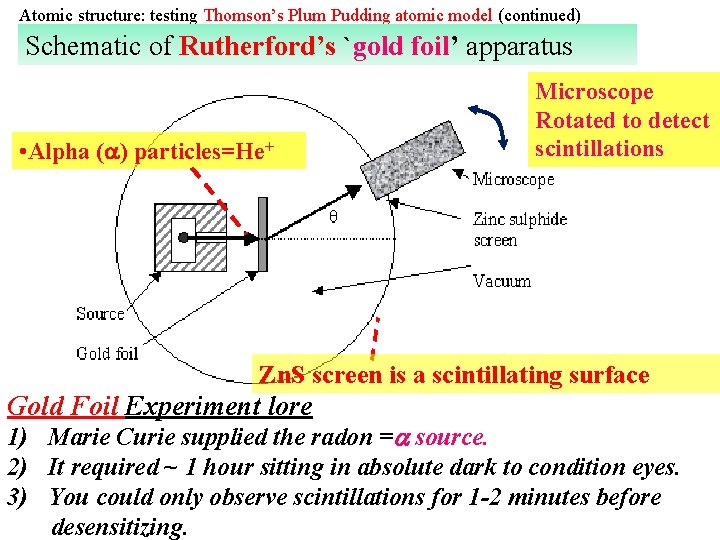
Atomic structure: testing Thomson’s Plum Pudding atomic model (continued) Schematic of Rutherford’s `gold foil’ apparatus • Alpha ( ) particles=He+ Microscope Rotated to detect scintillations Zn. S screen is a scintillating surface Gold Foil Experiment lore 1) Marie Curie supplied the radon = source. 2) It required ~ 1 hour sitting in absolute dark to condition eyes. 3) You could only observe scintillations for 1 -2 minutes before desensitizing.

Atomic structure: testing Thomson’s Plum Pudding Atomic Model(continued) Other Facts about the Gold Leaf Experiment rarely mentioned: • particles move crazy fast: velocity ~ 0. 1 c ~7*107 mph • particles are crazy overweight compared to the electrons (e-) in a gold atom: ~800 X heavier than all 79 e- in gold atom • The gold foil is crazy thin: ~ 8. 6*10 -6 cm thick (~1/3000 the thickness of cheap toilet paper )

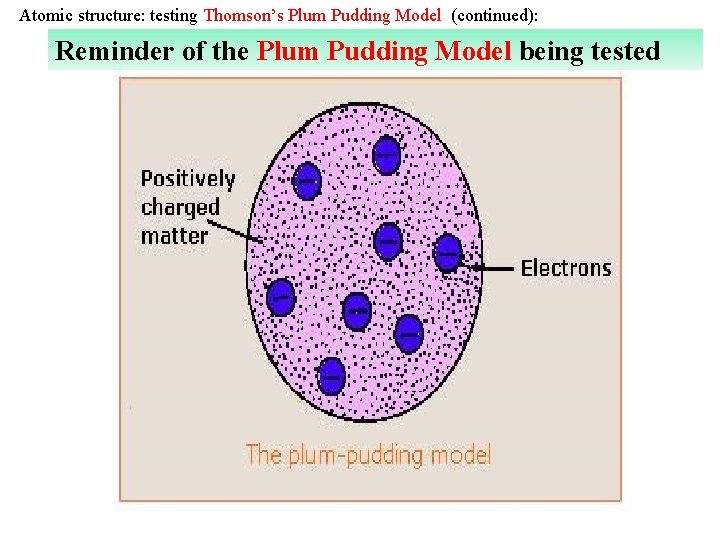
Atomic structure: testing Thomson’s Plum Pudding Model (continued): Reminder of the Plum Pudding Model being tested

Given the preceding facts, predict how the particles will behave after striking the gold foil if the structure of gold is as described in Thomson’s Plum Pudding model. A. Bounce straight backwards off the foil like a baseball hitting a wall. B. Punch through the foil like it wasn’t there. C. Scatter off the foil randomly in all directions.

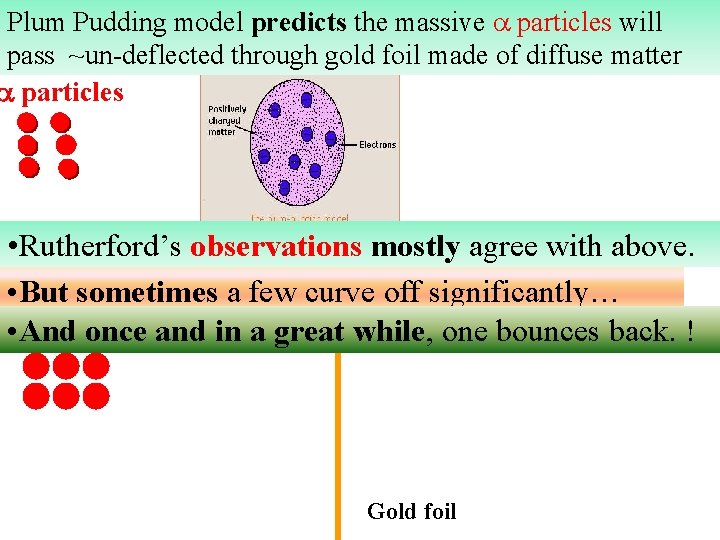
Plum Pudding model predicts the massive particles will pass ~un-deflected through gold foil made of diffuse matter particles • Rutherford’s observations mostly agree with above. • But sometimes a few curve off significantly… • And once and in a great while, one bounces back. ! Gold foil

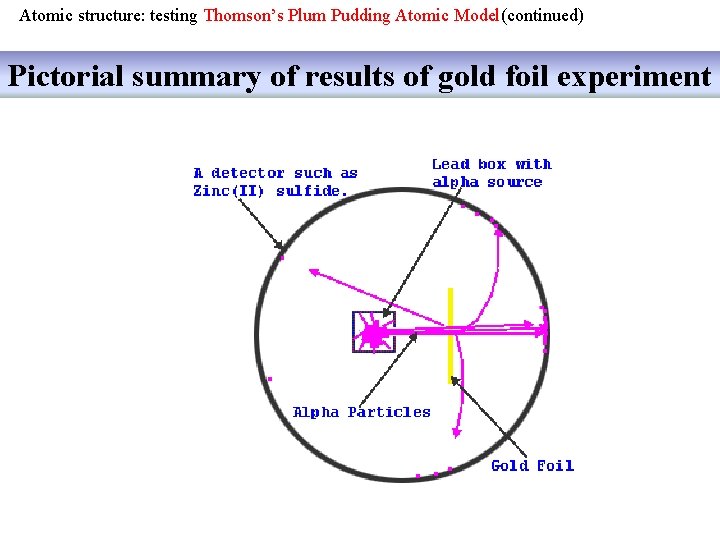
Atomic structure: testing Thomson’s Plum Pudding Atomic Model(continued) Pictorial summary of results of gold foil experiment

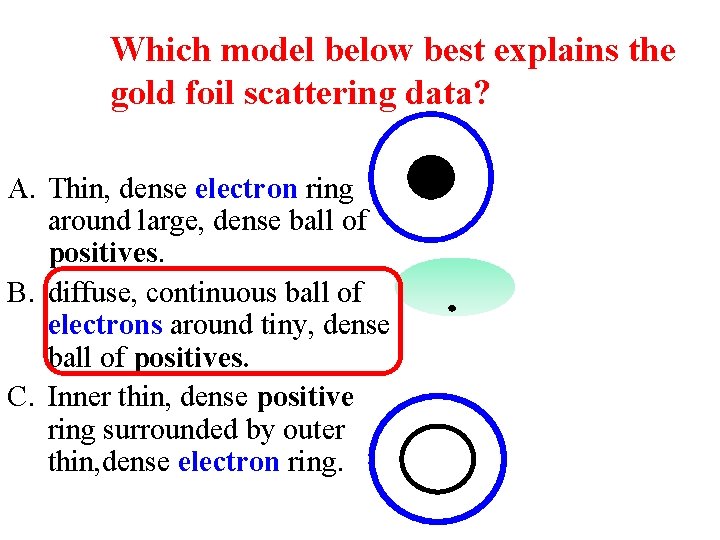
Which model below best explains the gold foil scattering data? A. Thin, dense electron ring around large, dense ball of positives. B. diffuse, continuous ball of electrons around tiny, dense ball of positives. C. Inner thin, dense positive ring surrounded by outer thin, dense electron ring.

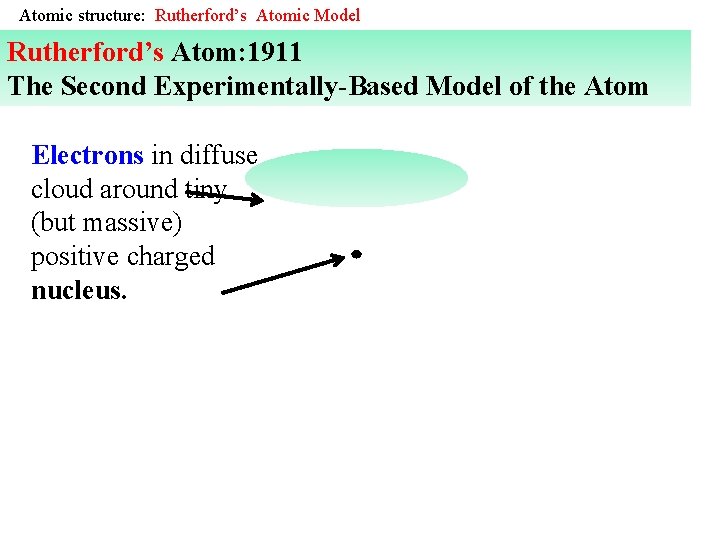
Atomic structure: Rutherford’s Atomic Model Rutherford’s Atom: 1911 The Second Experimentally-Based Model of the Atom Electrons in diffuse cloud around tiny (but massive) positive charged nucleus.

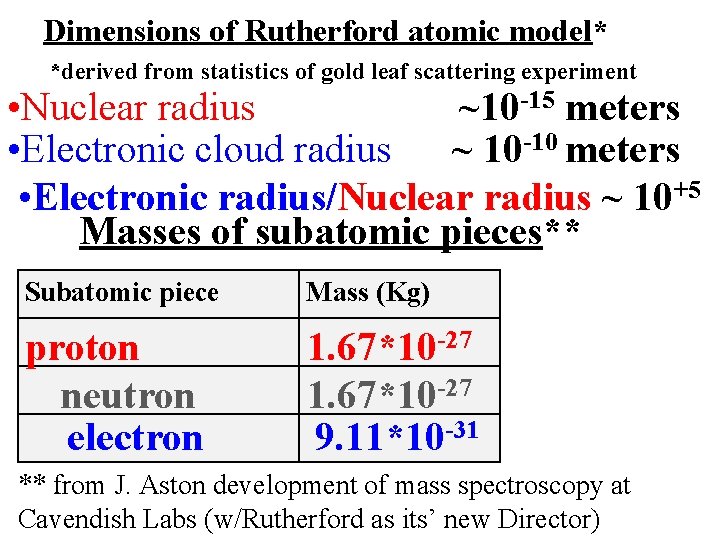
Dimensions of Rutherford atomic model* *derived from statistics of gold leaf scattering experiment • Nuclear radius ~10 -15 meters • Electronic cloud radius ~ 10 -10 meters • Electronic radius/Nuclear radius ~ 10+5 Masses of subatomic pieces** Subatomic piece Mass (Kg) proton neutron electron 1. 67*10 -27 9. 11*10 -31 ** from J. Aston development of mass spectroscopy at Cavendish Labs (w/Rutherford as its’ new Director)

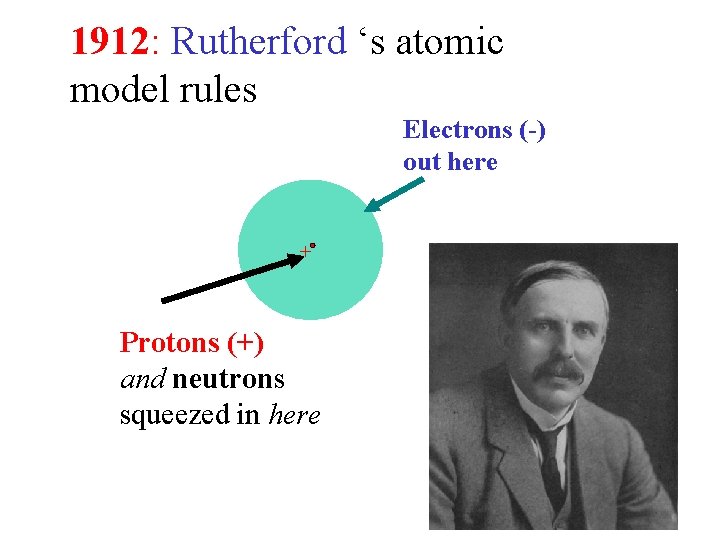
1912: Rutherford ‘s atomic model rules Electrons (-) out here + Protons (+) and neutrons squeezed in here

But there are 2 BIG Problems with Rutherford’s model 1)Why don’t the p+ and e- attract and come together ? ? ? (or…why isn’t Earth the size of a golf ball? ) ? ? ?

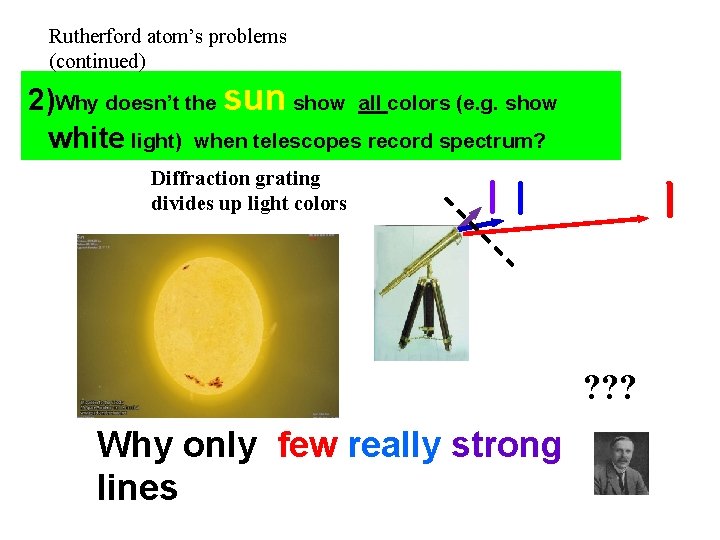
Rutherford atom’s problems (continued) 2)Why doesn’t the sun show all colors (e. g. show white light) when telescopes record spectrum? Diffraction grating divides up light colors ? ? ? Why only few really strong lines

BIGGER third AN EVEN PROBLEM FOR RUTHERFORD’S LAB 3) The photoelectric effect problem and the trouble with theory of light Help!!!!
 The frame size of a video refers to the video’s
The frame size of a video refers to the video’s Yandex video b****
Yandex video b**** Video.search.yahoo.com
Video.search.yahoo.com Videos yahoo search
Videos yahoo search Bellman ford algorithm
Bellman ford algorithm Francis ford selffinance 120m utopian
Francis ford selffinance 120m utopian Neil tang
Neil tang Bray v ford
Bray v ford Henry ford paternalistic leadership
Henry ford paternalistic leadership Which innovation helped henry ford realize his vision?
Which innovation helped henry ford realize his vision? Algoritmo di ford fulkerson
Algoritmo di ford fulkerson Bellman ford algorithm
Bellman ford algorithm Mmogle
Mmogle Eec test ford
Eec test ford Picture of dorian gray preface
Picture of dorian gray preface