RTOG 1308 Phase III Randomized Trial Comparing Overall










- Slides: 17

RTOG 1308: Phase III Randomized Trial Comparing Overall Survival after Photon versus Proton Chemoradiation Therapy for Inoperable Stage II-IIIB NSCLC PI: Zhongxing Liao

Co-Chairs Study Chair: Zhongxing Liao, MD Radiation Oncology: Jeffrey Bradley, MD, Washington University Noah Choi, MD, Massachusetts General Hospital Simone, Charles, MD, University of Pennsylvania Nguyen, Quyhn, MD Anderson Cancer Center Brad Hoppe, MD, University of Florida Eugen Hug, MD, Pro. Cure Medical Oncology: Charles Lu, MD Anderson Medical Physics: Michael Gillin, Ph. D, MD Anderson Radhe Mohan, Ph. D, MD Anderson Translational Research: Steven H. Lin, MD, Ph. D, MD Anderson Quality of Life: Xin Shelley Wang, MD Anderson Outcomes: Ben Movsas, MD, Henry Ford Health System Comparative Cost Effectiveness: Deborah Bruner, RN, Ph. D, Emory University Gregory Russo, MD, Boston University School of Medicine

Specific hypotheses • When standard constraints on radiation dose and volume to organs at risk (OARs) are used, proton beam therapy will allow lower doses to critical organs and higher doses to be delivered to the target compared with photons, which will result in an improvement of median survival time from 21 months (control arm) to 28 months (proton arm) in patients with good performance status and stage II-IIIB nonsmall cell lung cancer (NSCLC).

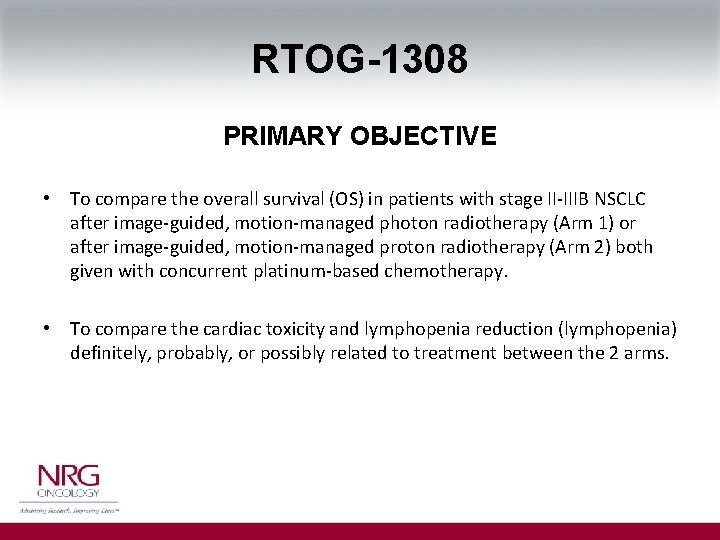
RTOG-1308 PRIMARY OBJECTIVE • To compare the overall survival (OS) in patients with stage II-IIIB NSCLC after image-guided, motion-managed photon radiotherapy (Arm 1) or after image-guided, motion-managed proton radiotherapy (Arm 2) both given with concurrent platinum-based chemotherapy. • To compare the cardiac toxicity and lymphopenia reduction (lymphopenia) definitely, probably, or possibly related to treatment between the 2 arms.

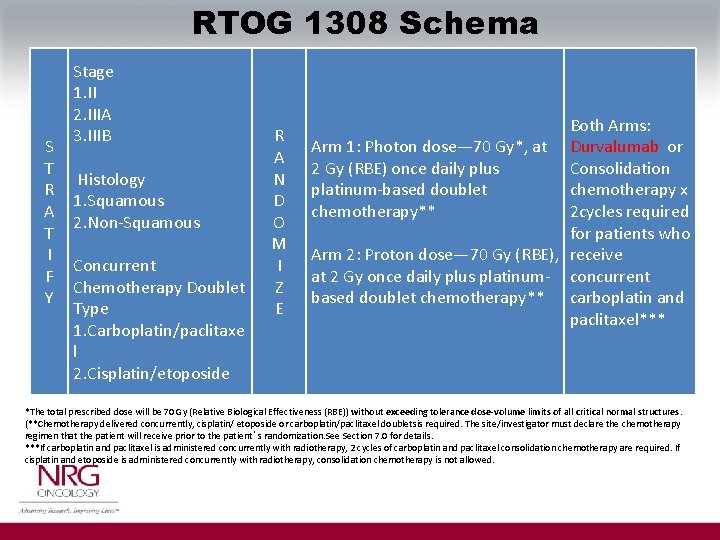
RTOG 1308 Schema S T R A T I F Y Stage 1. II 2. IIIA 3. IIIB Histology 1. Squamous 2. Non-Squamous Concurrent Chemotherapy Doublet Type 1. Carboplatin/paclitaxe l 2. Cisplatin/etoposide R A N D O M I Z E Both Arms: Arm 1: Photon dose— 70 Gy*, at Durvalumab or Consolidation 2 Gy (RBE) once daily plus platinum-based doublet chemotherapy x 2 cycles required chemotherapy** for patients who Arm 2: Proton dose— 70 Gy (RBE), receive at 2 Gy once daily plus platinum- concurrent based doublet chemotherapy** carboplatin and paclitaxel*** *The total prescribed dose will be 70 Gy (Relative Biological Effectiveness (RBE)) without exceeding tolerance dose-volume limits of all critical normal structures. (**Chemotherapy delivered concurrently, cisplatin/ etoposide or carboplatin/paclitaxel doubletsis required. The site/investigator must declare the chemotherapy regimen that the patient will receive prior to the patient’s randomization. See Section 7. 0 for details. ***If carboplatin and paclitaxel is administered concurrently with radiotherapy, 2 cycles of carboplatin and paclitaxel consolidation chemotherapy are required. If cisplatin and etoposide is administered concurrently with radiotherapy, consolidation chemotherapy is not allowed.

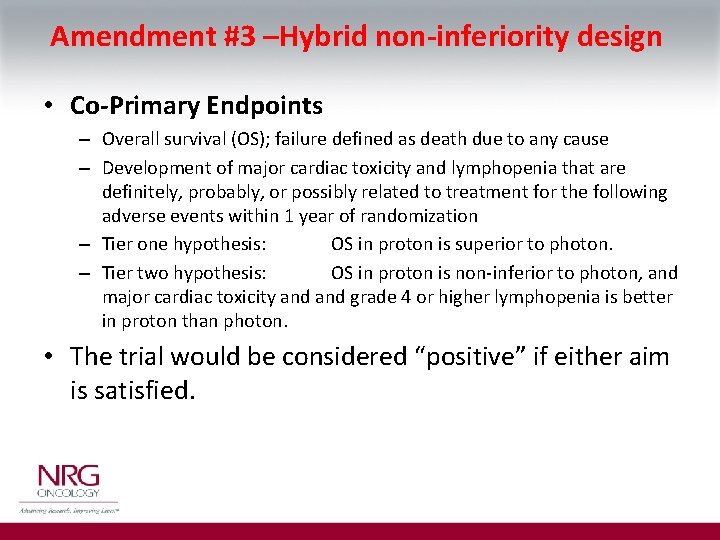
Amendment #3 –Hybrid non-inferiority design • Co-Primary Endpoints – Overall survival (OS); failure defined as death due to any cause – Development of major cardiac toxicity and lymphopenia that are definitely, probably, or possibly related to treatment for the following adverse events within 1 year of randomization – Tier one hypothesis: OS in proton is superior to photon. – Tier two hypothesis: OS in proton is non-inferior to photon, and major cardiac toxicity and grade 4 or higher lymphopenia is better in proton than photon. • The trial would be considered “positive” if either aim is satisfied.

Secondary Objectives • To compare 2 -year progression-free survival (PFS) between the 2 arms; • To compare the development of grade 3 or higher adverse events definitely, probably, or possibly related to treatment (see Section 13. 2 for specific adverse events). • To compare the development of patient-reported outcome – symptom burden [MDASI-Lung]), – Shortness Breath Questionnaire [SOBQ] – Health utility Euro. Qol [EQ 5 D] • To compare cost-effectiveness outcomes • To compare pulmonary function changes by treatment arms and response; • To explore the most appropriate and clinically relevant technological parameters to ensure quality and effectiveness (imaging, simulation, immobilization, target and critical structure definition, treatment planning, image guidance and delivery).

Amendment #3 - Eligibility • Allow pemetrxed to be given with platinum doublet • Allow prior induction systemic treatment (<= 4 cycles of induction chemotherapy or 6 month of targeted therapy) • Allow standard of care consolidation chemotherapy including immunotherapy (durvalumab, based on PACIFIC trial findings) • Recurrence after surgery only and the recommended therapy for the recurrence is concurrent chemoradiation therapy • Relax some of the lab eligibility criteria (e. g. PFT>=0. 8 L or >=35% predicted)

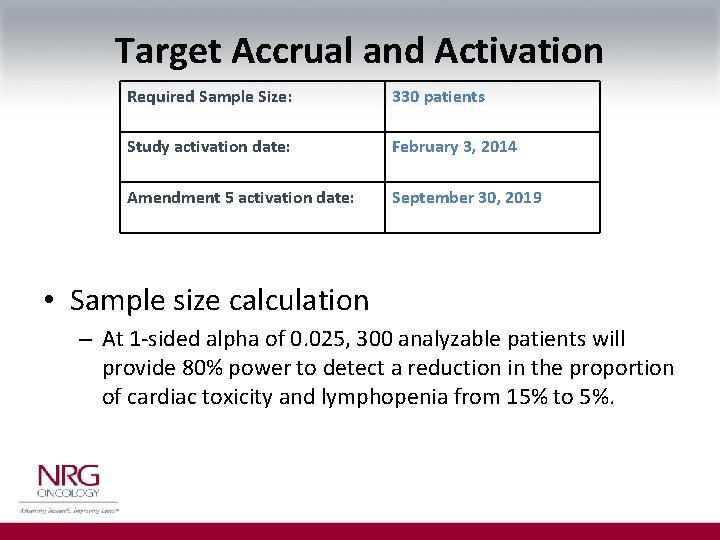
Target Accrual and Activation Required Sample Size: 330 patients Study activation date: February 3, 2014 Amendment 5 activation date: September 30, 2019 • Sample size calculation – At 1 -sided alpha of 0. 025, 300 analyzable patients will provide 80% power to detect a reduction in the proportion of cardiac toxicity and lymphopenia from 15% to 5%.

Accrual Goals • The monthly accrual goal is 6 -7 patients per month. • Actual monthly accrual rate – For the life of the study = 2. 5/month – For the last 6 months = 3. 5/month • The number of credentialed sites continued to grow, meanwhile the efforts have been partially dampened due to COVID-19 pandemic. • CTEP has been monitoring the accrual for the study, and planned to reevaluate the accrual rate again in April 2020. • There is no set goal from CTEP, but ideally accrual needs to be greater than 50% of projected, which would be at least 4 patients per month.

CTEP Letter 1 -2020 Dear Dr Curran, CTEP/NCI has considered your request to continue NRG-RTOG 1308, Phase III Randomized Trial Comparing Overall Survival After Photon Versus Proton Chemoradiotherapy for Inoperable Stage II-IIIB NSCLC. Based on the strategies that NRG will undertake to address accrual issues, it was decided to continue the trial for one more year to catch up with the accrual and monitor it closely.

Monthly Conference Call Agenda 1. Roll call – NRG staff 2. Study overview - Liao 3. Monthly report that shows current accrual (Becky/Chen update each month) 4. Review accruals 5. Review what sites specific barriers and share experiences 6. Review AEs, planning issues 7. Discussion on data collection, missing data or delinquent submission 8. Etc….

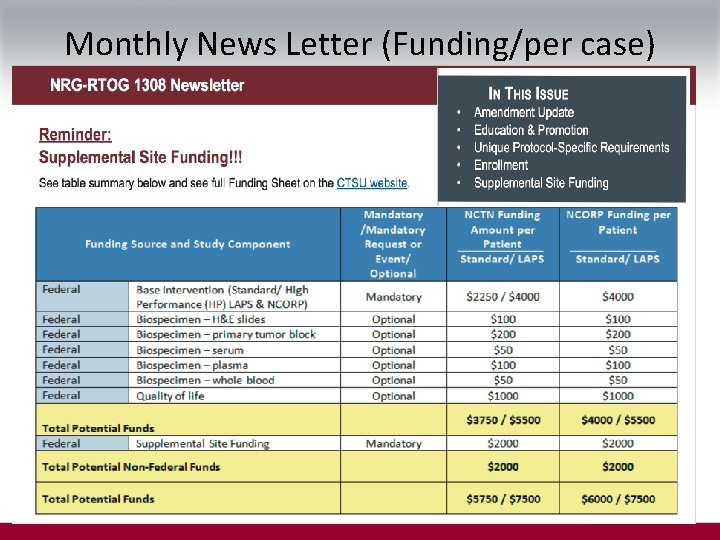
Monthly News Letter (Funding/per case)

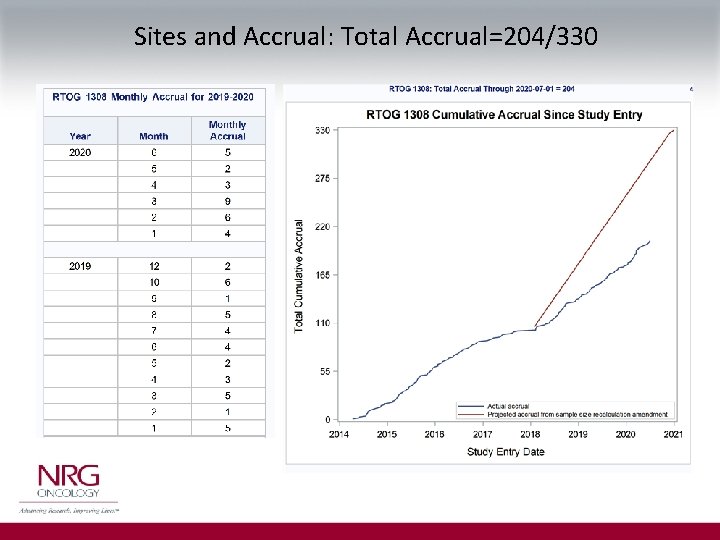
Sites and Accrual: Total Accrual=204/330

Every patient counts!

Challenges • Accrual, accrual! - goal 5 patient/month, we need ALL participating centers to enroll one patient per month!

RTOG 1308: A historical opportunity for our specialty! Let’s Do It!