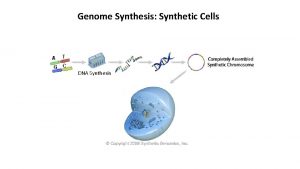
Revision of C 6 Chemical synthesis Chemical synthesis











- Slides: 29

Revision of C 6 Chemical synthesis

Chemical synthesis: chemical reactions and processes used to get a desired product using starting materials called reagents. The products can be useful for a variety of purposes but tend to be … To provide food additives, fertilisers, dyestuffs, paints, pigments and pharmaceuticals. • fine chemicals • bulk chemicals

Recall the formulae of …. Gases: chlorine = Cl 2, hydrogen = H 2, nitrogen= N 2, oxygen = O 2 Acids: hydrochloric acid = HCl, nitric acid = HNO 3, sulfuric acid = H 2 SO 4, Alkalis • sodium hydroxide = Na. OH, • magnesium hydroxide Mg(OH)2

Salts sodium chloride = Na. Cl, magnesium oxide = Mg. O, potassium chloride = KCl calcium chloride = Ca. Cl 2, More complex salts… magnesium carbonate = [Mg. CO 3] magnesium sulfate = [Mg. SO 4]; sodium carbonate [Na 2 CO 3]; calcium carbonate [Ca. CO 3]

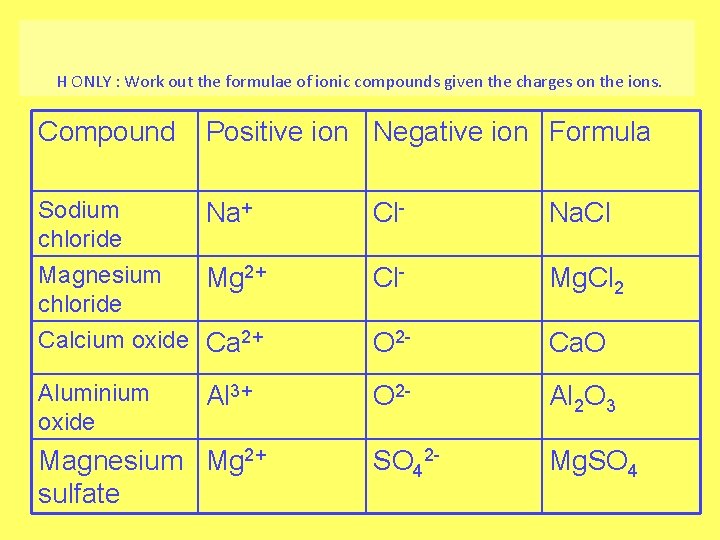
H ONLY : Work out the formulae of ionic compounds given the charges on the ions. Compound Positive ion Negative ion Formula Sodium chloride Magnesium chloride Na+ Cl- Na. Cl Mg 2+ Cl- Mg. Cl 2 Calcium oxide Ca 2+ O 2 - Ca. O Aluminium oxide O 2 - Al 2 O 3 SO 42 - Mg. SO 4 Al 3+ Magnesium Mg 2+ sulfate

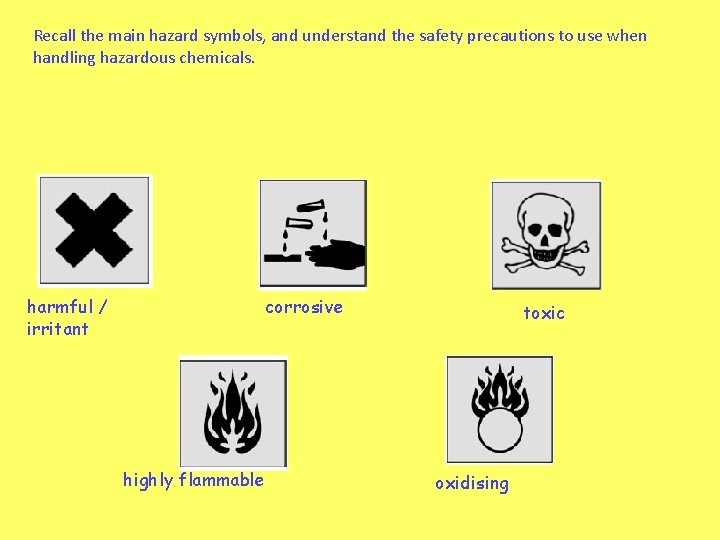
Recall the main hazard symbols, and understand the safety precautions to use when handling hazardous chemicals. harmful / irritant corrosive highly flammable toxic oxidising

Examples of pure acidic compounds which are solid, liquids and gases. Solids = citric acid & tartaric acid Liquids = sulfuric, nitric and ethanoic acids Gases = hydrogen chloride Recall common alkalis… Ø sodium hydroxide Ø potassium hydroxide Ø calcium hydroxide.

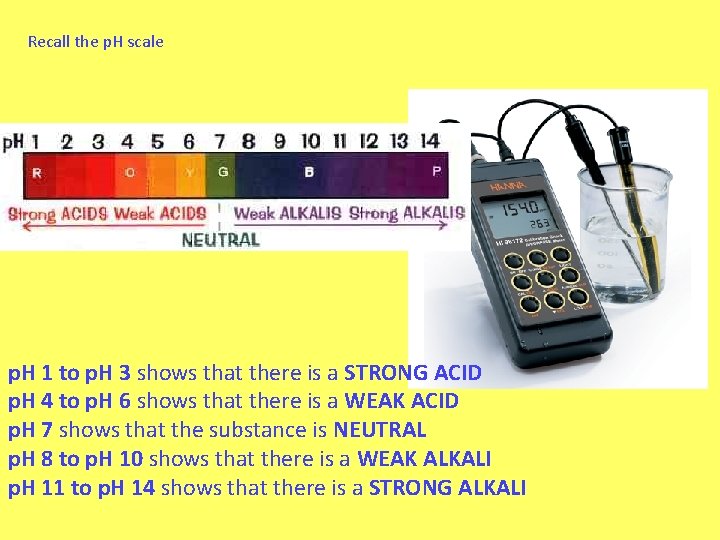
Recall the p. H scale p. H 1 to p. H 3 shows that there is a STRONG ACID p. H 4 to p. H 6 shows that there is a WEAK ACID p. H 7 shows that the substance is NEUTRAL p. H 8 to p. H 10 shows that there is a WEAK ALKALI p. H 11 to p. H 14 shows that there is a STRONG ALKALI

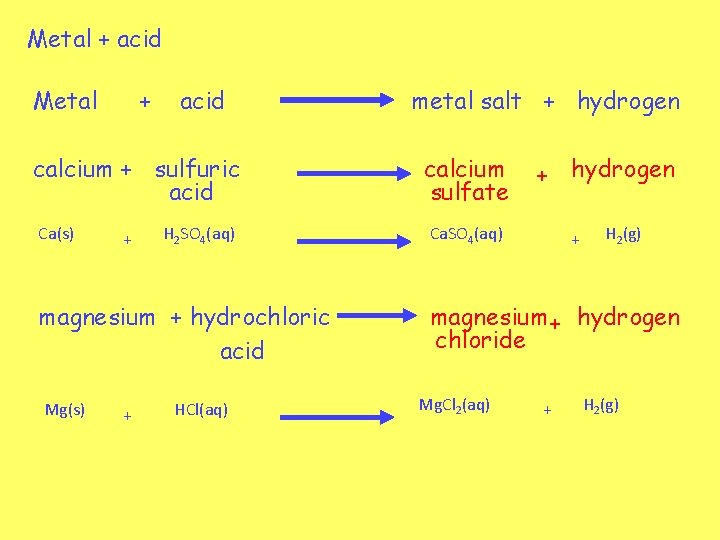
Metal + acid calcium + sulfuric acid Ca(s) + H 2 SO 4(aq) magnesium + hydrochloric acid Mg(s) + HCl(aq) metal salt + hydrogen calcium sulfate + hydrogen Ca. SO 4(aq) + H 2(g) magnesium + hydrogen chloride Mg. Cl 2(aq) + H 2(g)

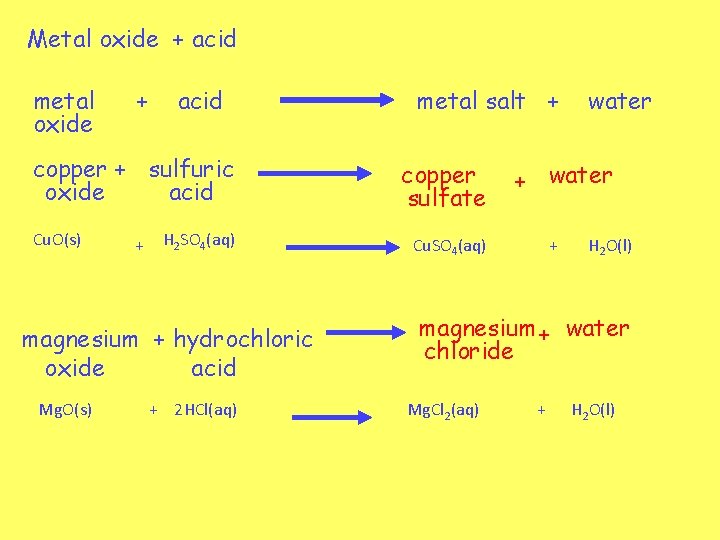
Metal oxide + acid metal oxide + acid copper + sulfuric oxide acid Cu. O(s) + H 2 SO 4(aq) magnesium + hydrochloric oxide acid Mg. O(s) + 2 HCl(aq) metal salt + copper sulfate water + water Cu. SO 4(aq) + H 2 O(l) magnesium + water chloride Mg. Cl 2(aq) + H 2 O(l)

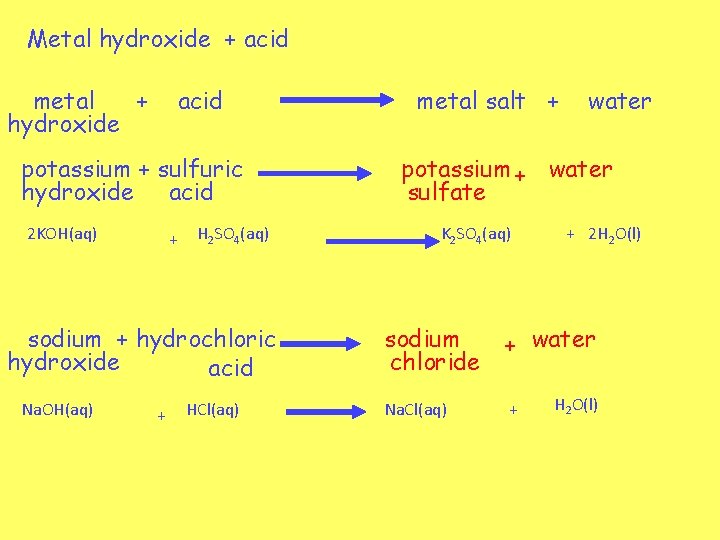
Metal hydroxide + acid metal + hydroxide acid potassium + sulfuric hydroxide acid 2 KOH(aq) + H 2 SO 4(aq) sodium + hydrochloric hydroxide acid Na. OH(aq) + HCl(aq) metal salt + water potassium + water sulfate K 2 SO 4(aq) + 2 H 2 O(l) sodium chloride + water Na. Cl(aq) + H 2 O(l)

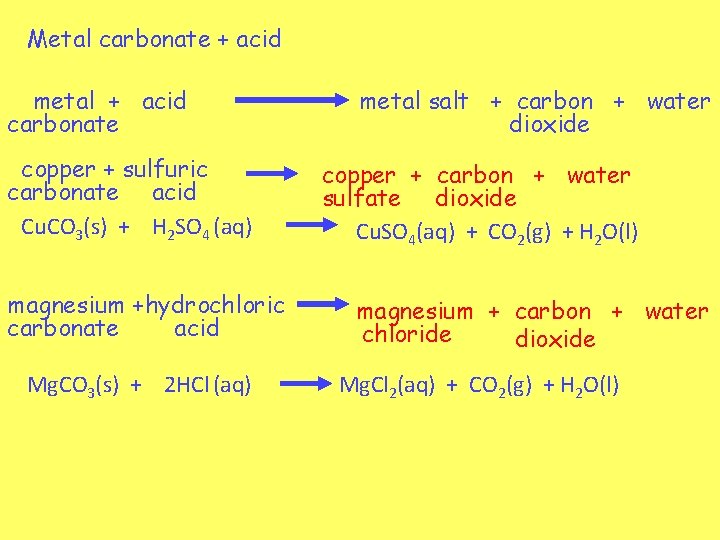
Metal carbonate + acid metal + acid carbonate copper + sulfuric carbonate acid Cu. CO 3(s) + H 2 SO 4 (aq) magnesium +hydrochloric carbonate acid Mg. CO 3(s) + 2 HCl (aq) metal salt + carbon + water dioxide copper + carbon + water sulfate dioxide Cu. SO 4(aq) + CO 2(g) + H 2 O(l) magnesium + carbon + water chloride dioxide Mg. Cl 2(aq) + CO 2(g) + H 2 O(l)

Reaction of acid with an alkali to form a salt is a neutralisation reaction. H+ ions from the acid react with OH- ions from the alkali. • When the number of H+ ions is exactly matched by the number of OH- ions to form a p. H of 7 • H+(aq) + OH- (aq) H 2 O(l) • An alkali cancel out an acid to form a salt and the water (shown above)

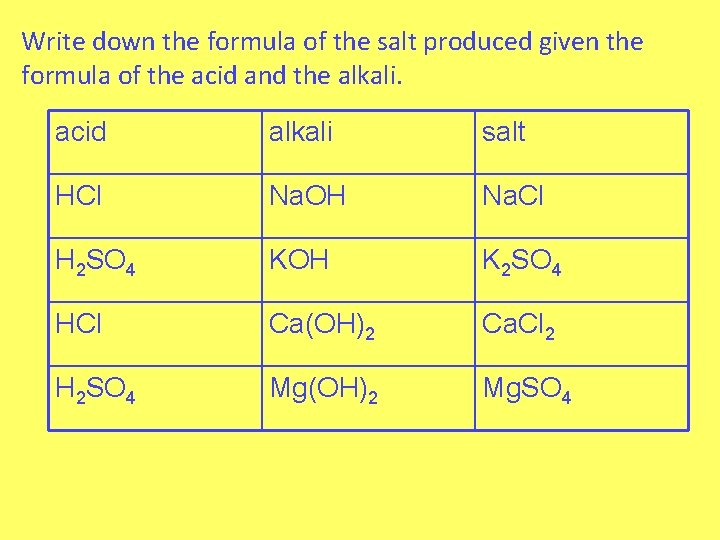
Write down the formula of the salt produced given the formula of the acid and the alkali. acid alkali salt HCl Na. OH Na. Cl H 2 SO 4 KOH K 2 SO 4 HCl Ca(OH)2 Ca. Cl 2 H 2 SO 4 Mg(OH)2 Mg. SO 4

C 6. 2 : Planning, Carrying out and controlling chemical synthesis 1. Identify the stages in the chemical synthesis of an inorganic compound. • • choosing the reaction or series of reactions risk assessment (chemical and procedural) working out the quantities of reactants to use carrying out the reaction in suitable apparatus in the right conditions (such as temperature, concentration or the presence of a catalyst) • separating the product from the reaction mixture • purifying the product • measuring the yield and checking the purity of the product.

Understand the purpose of these techniques…. • Dissolving… forming solutions to allow easy mixing of reactants • Crystallisation… to purify a sample by the formation of pure crystals from a cooled (often saturated) solution, • Filtration… to separate solid impurities from a solution, or to remove excess solid. • Evaporation… to remove excess solvent from a solution • Drying in an oven or dessicator… to remove water without the risk of wasting yield. • Titration… to find the concentration of an acid (or alkali) using an alkali (or acid) of a known concentration AND an indicator

Balancing equations Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) +H 2(g) Reactants : 1 x Magnesium atom 2 x Hydrogen atoms 2 x Chlorine atoms

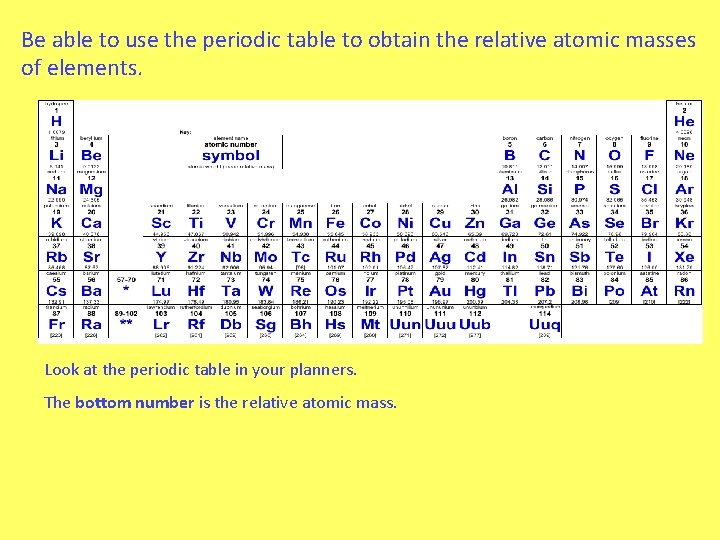
Be able to use the periodic table to obtain the relative atomic masses of elements. Look at the periodic table in your planners. The bottom number is the relative atomic mass.

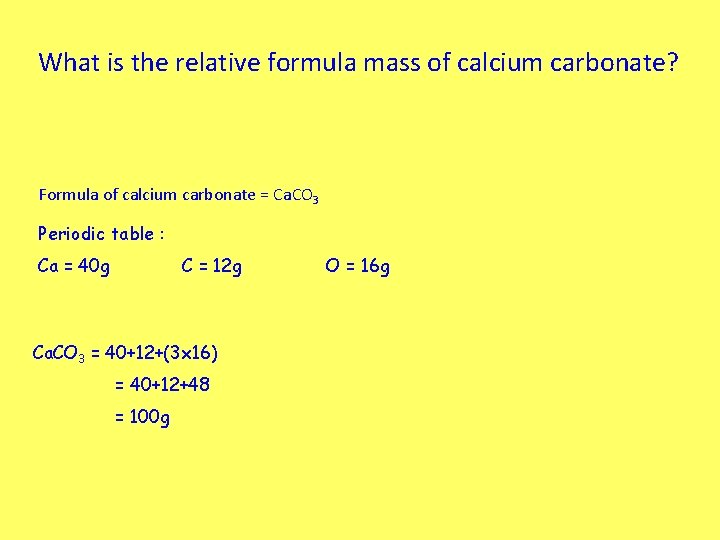
What is the relative formula mass of calcium carbonate? Formula of calcium carbonate = Ca. CO 3 Periodic table : Ca = 40 g C = 12 g Ca. CO 3 = 40+12+(3 x 16) = 40+12+48 = 100 g O = 16 g

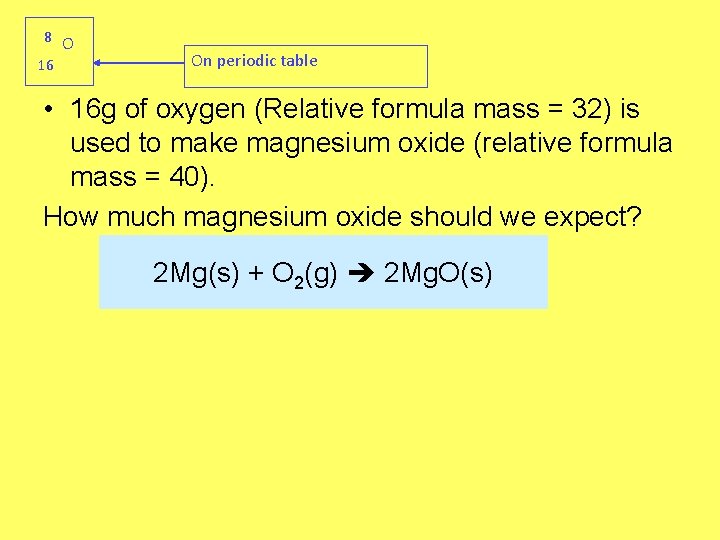
8 O 16 On periodic table • 16 g of oxygen (Relative formula mass = 32) is used to make magnesium oxide (relative formula mass = 40). How much magnesium oxide should we expect? 2 Mg(s) + O 2(g) 2 Mg. O(s)

• 2. 4 g of magnesium (Relative atomic mass = 24) is used to make magnesium chloride (relative formula mass = 95). How much of this salt should we expect? Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) +H 2(g)

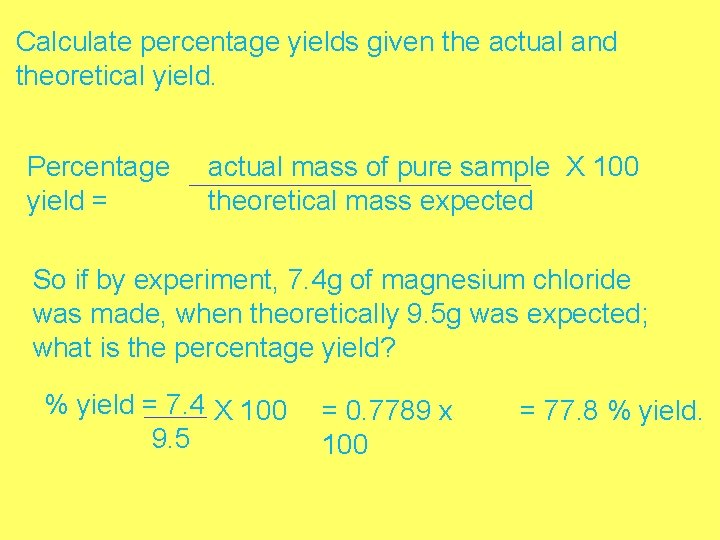
Calculate percentage yields given the actual and theoretical yield. Percentage yield = actual mass of pure sample X 100 theoretical mass expected So if by experiment, 7. 4 g of magnesium chloride was made, when theoretically 9. 5 g was expected; what is the percentage yield? % yield = 7. 4 X 100 9. 5 = 0. 7789 x 100 = 77. 8 % yield.

Titrations An acid-base titration is the determination of the concentration of an acid or base by exactly neutralizing the acid/base with an acid or base of known concentration. Acid-Base titrations can also be used to find percent purity of chemicals.

Rate of reaction If it is too fast it could make it unsafe. (eg could get too hot if exothermic; gas could be produced to quickly and pressure build up) If it is too slow, then product would be made too slowly, and yield low, so profit too low. (economic factors) Recall that reaction rates vary with…. • particle size (surface area) • concentration • temperature.

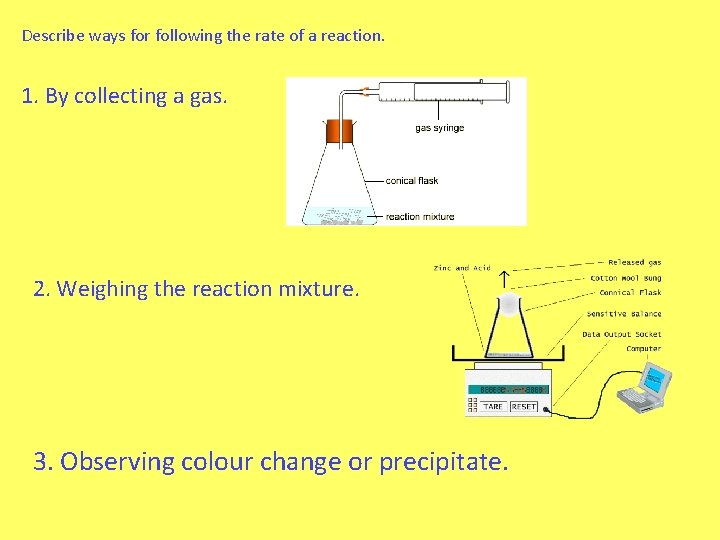
Describe ways for following the rate of a reaction. 1. By collecting a gas. 2. Weighing the reaction mixture. 3. Observing colour change or precipitate.

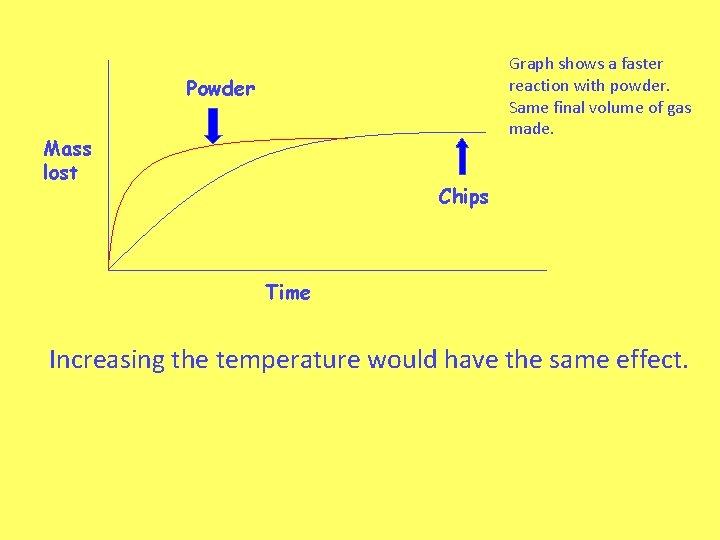
Graph shows a faster reaction with powder. Same final volume of gas made. Powder Mass lost Chips Time Increasing the temperature would have the same effect.

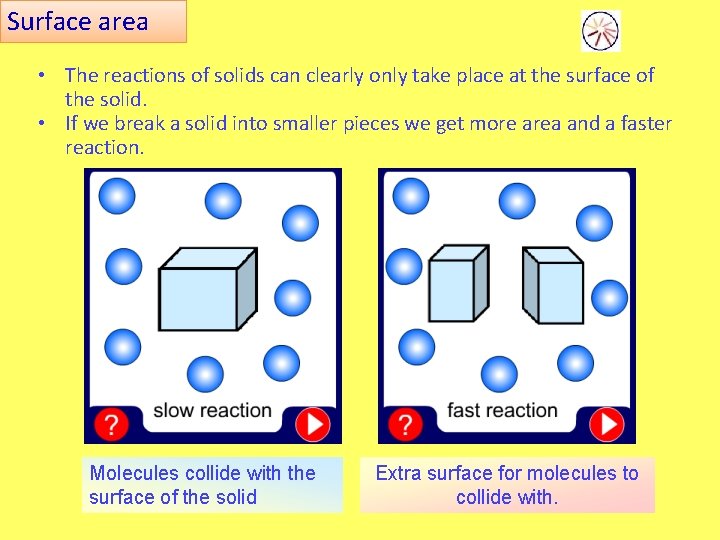
Surface area • The reactions of solids can clearly only take place at the surface of the solid. • If we break a solid into smaller pieces we get more area and a faster reaction. Molecules collide with the surface of the solid Extra surface for molecules to collide with.

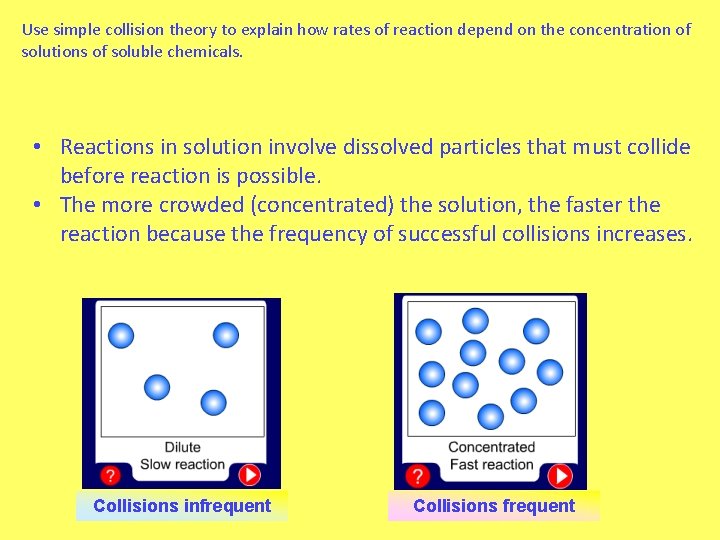
Use simple collision theory to explain how rates of reaction depend on the concentration of solutions of soluble chemicals. • Reactions in solution involve dissolved particles that must collide before reaction is possible. • The more crowded (concentrated) the solution, the faster the reaction because the frequency of successful collisions increases. Collisions infrequent Collisions frequent

Understand that catalysts speed up a chemical reaction while not being used up in the process. Because they are not used up, they are recyclable. For chemical reactions to occur: • Existing bonds have to begin breaking so that new ones can be formed. • The molecules have to collide in such a way that the reacting parts of the molecules are brought together. Catalysts can help with either or both of these processes.
 Revision passive voice
Revision passive voice Example of double replacement reaction
Example of double replacement reaction Synthesis chemical equation
Synthesis chemical equation Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 7 chemical formulas and chemical compounds
Chapter 7 chemical formulas and chemical compounds Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Are kc and kp equal
Are kc and kp equal Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Preposition examples
Preposition examples Ict igcse practical revision presentation
Ict igcse practical revision presentation Tan chord theorem proof
Tan chord theorem proof Wjec criminology unit 4 past papers
Wjec criminology unit 4 past papers Jekyll and hyde revision
Jekyll and hyde revision Freytag's pyramid othello
Freytag's pyramid othello Ebbinghaus formula
Ebbinghaus formula Ict igcse theory revision presentation
Ict igcse theory revision presentation Anuradha perera.lk final revision
Anuradha perera.lk final revision Gcse pe revision booklet
Gcse pe revision booklet Kinesthetic learning
Kinesthetic learning Gasrocnemius
Gasrocnemius Rcgp akt practice questions
Rcgp akt practice questions Animal farm revision guide
Animal farm revision guide Work area protection manual
Work area protection manual Year 9 french revision booklet
Year 9 french revision booklet Sailmaker by alan spence
Sailmaker by alan spence Half my life is an act of revision
Half my life is an act of revision Mathsrevision.com
Mathsrevision.com Mathsrevision.com
Mathsrevision.com Tenses englisch
Tenses englisch