Review of regulatory capacity a strategy to strengthen
































- Slides: 45

Review of regulatory capacity: a strategy to strengthen national authorities Valerio Reggi, Eshetu Wondemagegnehu, HTP/EDM/QSM 1 October 2003 1 PAR Seminar 1 October 2003 WHO - HTP

Outline of presentation • Background • Strengthening national authorities 2 PAR Seminar 1 October 2003 WHO - HTP

Review of regulatory capacity Background Review of national regulatory situation 3 PAR Seminar 1 October 2003 WHO - HTP

World Health Assembly resolution 52. 19 24 May 1999: WHO’s Revised Drug Strategy URGES Member States: (1) to reaffirm their commitment to …… taking all necessary concrete measures in order to ensure equitable access to essential drugs; (2) to ensure that public health interests are paramount in pharmaceutical and health policies; (3) to explore and review their options under relevant international agreements, including trade agreements, to safeguard access to essential drugs; 4 PAR Seminar 1 October 2003 WHO - HTP

World Health Assembly resolutions & statement Implications for national regulatory authorities: - put public health as first priority of regulatory system development strategy and decisions - contribute to develop market of good quality, safe, effective, and affordable drugs and vaccines - improve regulatory assessment and market control Improve regulatory system's capacity to meet needs of health decision makers , professionals and public 5 PAR Seminar 1 October 2003 WHO - HTP

Consequences of weak regulatory capacity n n Irrational consumption and prescription Substandard, counterfeit, harmful, useless drugs and vaccines on sale 6 PAR Seminar 1 October 2003 WHO - HTP

Regulation of drugs and vaccines is an essential public-sector function n n Equity: who cares for the poor? Information imbalance: access to and capacity to assess information on quality, safety, efficacy, value for money, appropriateness n External benefits: immunizations and treatment of contagious diseases benefit all, if left to market laws alone many will not be immunized or treated 7 PAR Seminar 1 October 2003 WHO - HTP

Key elements of drug regulatory system Drug regulation comprises all the legal, administrative & technical arrangements meant to ensure that: n n n all premises, persons & practices engaged in the development, manufacture, importation, exportation, wholesale, supply, dispensing & promotion of drugs comply with approved standards, norms, procedures and requirements drug products are safe, effective and of acceptable quality product information is unbiased, accurate and appropriate drugs are available drugs are used rationally 8 PAR Seminar 1 October 2003 WHO - HTP

Key elements of drug regulatory system Basic functions in drug regulation (1) n n Licensing of manufacturers, importers, distributors, wholesale and retail outlets (premises, persons and practices) Marketing authorization for drug products Quality control laboratory testing Provision of drug information and monitoring of drug promotion and advertising Continues……. . . 9 PAR Seminar 1 October 2003 WHO - HTP

Key elements of drug regulatory system …. continued n n n Basic functions in drug regulation (2) Inspection of manufacturing and distribution channel premises Adverse drug reaction monitoring Authorization of clinical trials Monitoring of drug dispensing and prescribing practices Monitoring of drug utilization and promotion of rational drug use Application of sanctions 10 PAR Seminar 1 October 2003 WHO - HTP

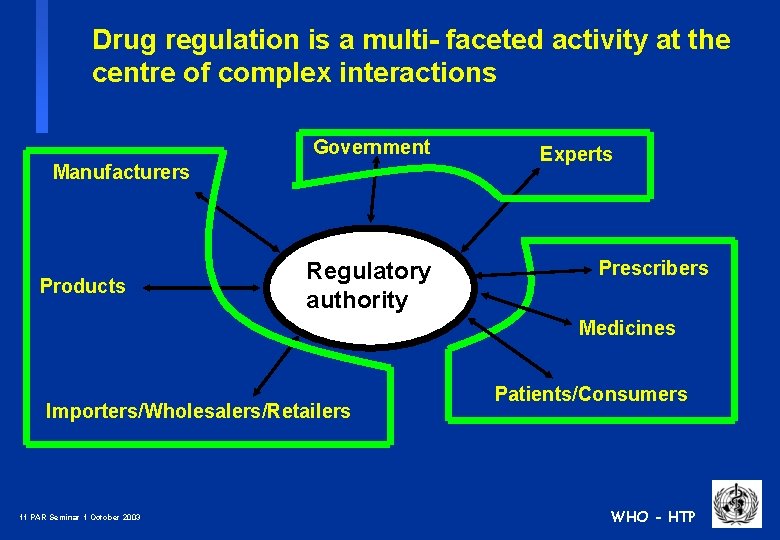
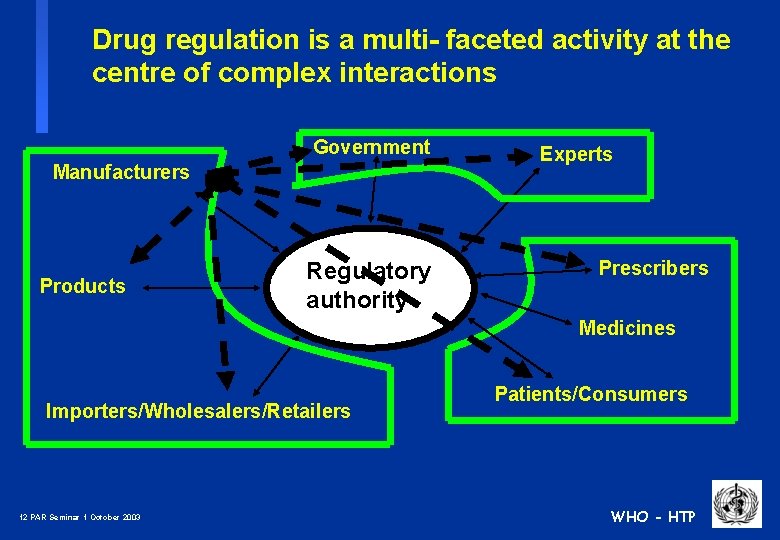
Drug regulation is a multi- faceted activity at the centre of complex interactions Government Manufacturers Products Regulatory authority Experts Prescribers Medicines Importers/Wholesalers/Retailers 11 PAR Seminar 1 October 2003 Patients/Consumers WHO - HTP

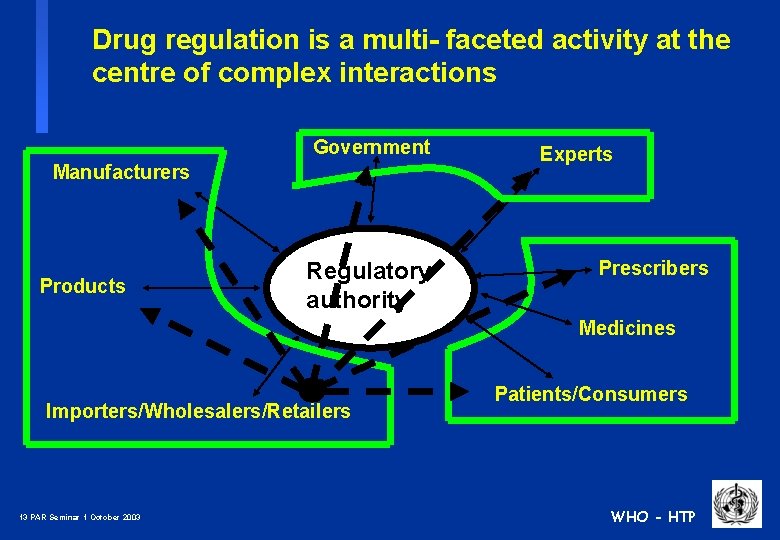
Drug regulation is a multi- faceted activity at the centre of complex interactions Government Manufacturers Products Regulatory authority Experts Prescribers Medicines Importers/Wholesalers/Retailers 12 PAR Seminar 1 October 2003 Patients/Consumers WHO - HTP

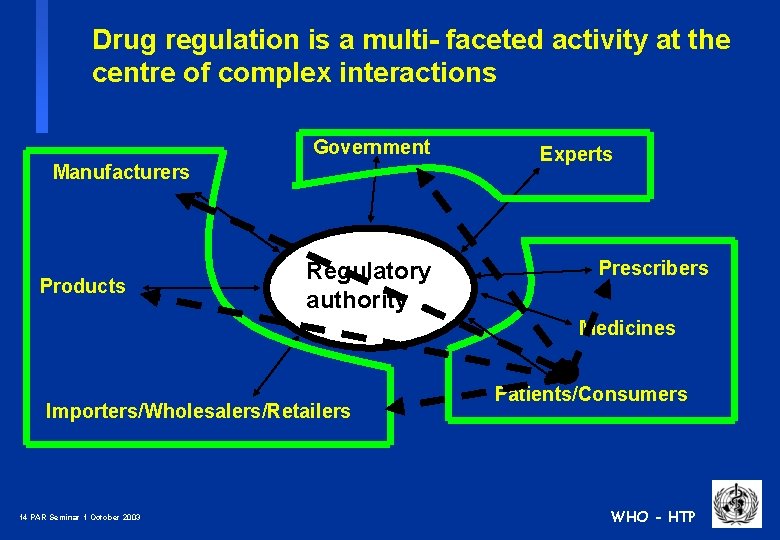
Drug regulation is a multi- faceted activity at the centre of complex interactions Government Manufacturers Products Regulatory authority Experts Prescribers Medicines Importers/Wholesalers/Retailers 13 PAR Seminar 1 October 2003 Patients/Consumers WHO - HTP

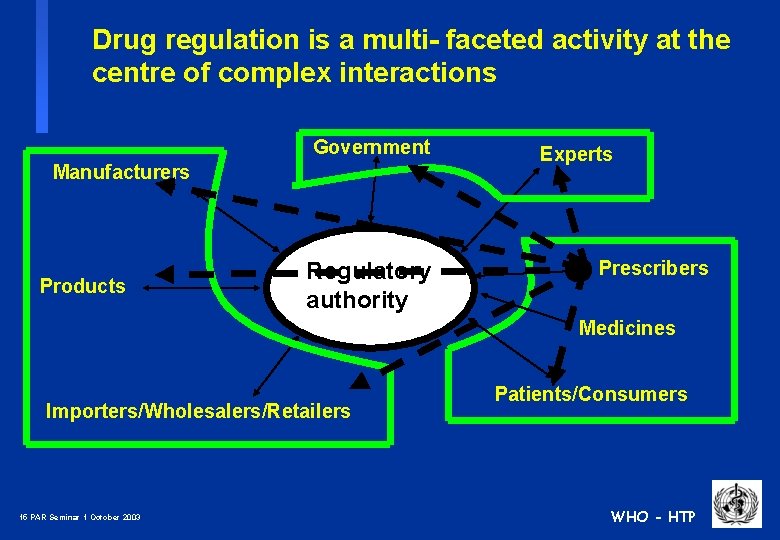
Drug regulation is a multi- faceted activity at the centre of complex interactions Government Manufacturers Products Regulatory authority Experts Prescribers Medicines Importers/Wholesalers/Retailers 14 PAR Seminar 1 October 2003 Patients/Consumers WHO - HTP

Drug regulation is a multi- faceted activity at the centre of complex interactions Government Manufacturers Products Regulatory authority Experts Prescribers Medicines Importers/Wholesalers/Retailers 15 PAR Seminar 1 October 2003 Patients/Consumers WHO - HTP

The challenges: n the health system counts on DRA for good, safe, and effective medicines and for fair rules and control on drug trade, information, and use n any strategy to improve anything in the pharmaceutical area involves DRA n any problem encountered in the pharmaceutical area has something to do with the DRA 16 PAR Seminar 1 October 2003 WHO - HTP

Country strategies for achieving effective drug regulation 1. Assess drug regulation performance 2. Identify and develop priority functions 3. Provide clear mission and purpose 4. Create a supportive environment 5. Formulate adequate legislation 6. Create appropriate organisational structure 7. Allocate adequate human and financial resources 8. Minimise corruption and conflict of interest 9. Apply appropriate regulatory & enforcement strategies 17 PAR Seminar 1 October 2003 WHO - HTP

Effective regulation depends on the environment n n n n adequate consideration is given to the public health value of drugs norms and regulations are locally meaningful drug regulation is not fragmented drug regulation processes are transparent DRA is also accountable to the public appropriate sanctions are regularly applied strong, organised public interest groups are encouraged to play a role in the drug regulatory area there is freedom of association and information 18 PAR Seminar 1 October 2003 WHO - HTP

Drug regulation and public health Good regulatory practice requires: § § § Mission and objectives clearly stated Possibility to assess attainment of objectives Procedures and outcomes transparent to applicants, health professionals, and public Arguments used to reach decision accessible to the public Reasonable duration of assessment without compromising quality, safety & efficacy 9 th ICDRA 29/4/1999 19 PAR Seminar 1 October 2003 WHO - HTP

Drug regulation and public health Good regulatory practice requires: § § Expedite review for orphan and outstanding public-health-value drugs Accountability to government, those regulated, and the public Personnel adequately trained, highly qualified, of high integrity Human resource development programme 9 th ICDRA 29/4/1999 20 PAR Seminar 1 October 2003 WHO - HTP

Drug regulation and public health Good regulatory practice requires: § § Mechanisms for appeal and for citizens' complaints Access to appropriate knowledge and technology Citizens are provided with accurate and appropriate drug information Mechanisms to ensure quality of operating procedures 9 th ICDRA 29/4/1999 21 PAR Seminar 1 October 2003 WHO - HTP

Drug regulation and public health No national authority meets all criteria, . . . but some are closer than others All want to improve, . . but some are much better placed 22 PAR Seminar 1 October 2003 WHO - HTP

Big gaps exist 23 PAR Seminar 1 October 2003 WHO - HTP

Thousands of highly qualified professionals Thousands of highly qualified external experts Virtually unlimited access to the most sophisticated technology and knowledge 24 PAR Seminar 1 October 2003 WHO - HTP

What is the best approach to strengthening national regulatory authorities? 25 PAR Seminar 1 October 2003 WHO - HTP

WHO Expert Committee on Specifications for Pharmaceutical Preparations, TSR 790, 1990: . . . approach to regulation must be attuned to available resources. . . problems in establishing regulatory control have too often resulted from the introduction of provisions successful elsewhere but of a complexity that precludes their effective implementation in the country of adoption. . . 26 PAR Seminar 1 October 2003 WHO - HTP

No importable models Need for review of national regulatory situation and definition of country-specific strategy and priorities 27 PAR Seminar 1 October 2003 WHO - HTP

WHO's approach: n n n develop policies tools for review of regulatory situation "how to" manuals training independent advice support to regional technical cooperation initiatives 28 PAR Seminar 1 October 2003 WHO - HTP

Review of regulatory capacity Background Review of national regulatory situation 29 PAR Seminar 1 October 2003 WHO - HTP

Review of regulatory situation Most elements are common to drugs and vaccines n A single national authority n Same legal basis n Same basic infrastructure & expertise n Same public-health values n Same need to ensure close links with health system n Same need to assess quality, safety and efficacy n Same need to ensure effective market control 30 PAR Seminar 1 October 2003 WHO - HTP

Review of national situation Only a few issues specific to either drugs or vaccines Vaccines Lot release 31 PAR Seminar 1 October 2003 Drugs Generics Drug information & promotion WHO - HTP

Key issues n Identify general indicators and key NRA functions n Develop data collection tool that permits to obtain information enabling to identify areas of strength and weakness n Develop criteria to review information collected and turn findings into recommendations 32 PAR Seminar 1 October 2003 WHO - HTP

General indicators Regulatory functions

Review tool Function Component Examples & Criteria Component . . . . Examples & Criteria . . . .

Regulatory functions

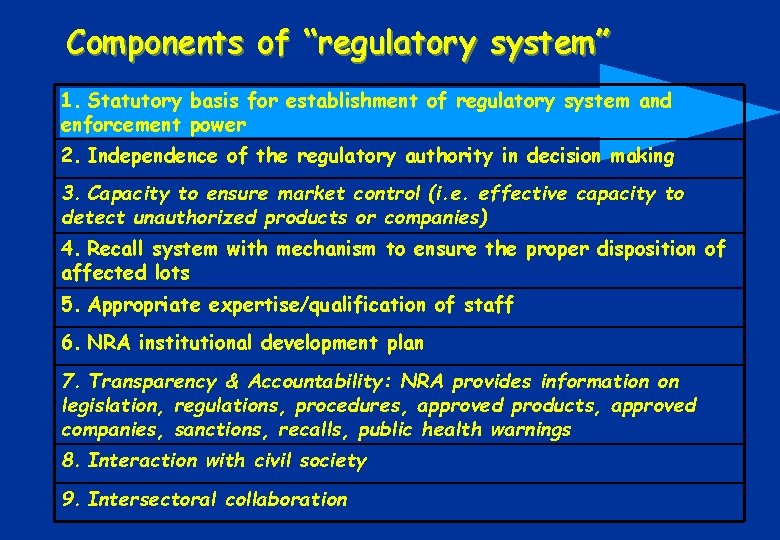
Components of “regulatory system” 1. Statutory basis for establishment of regulatory system and enforcement power 2. Independence of the regulatory authority in decision making 3. Capacity to ensure market control (i. e. effective capacity to detect unauthorized products or companies) 4. Recall system with mechanism to ensure the proper disposition of affected lots 5. Appropriate expertise/qualification of staff 6. NRA institutional development plan 7. Transparency & Accountability: NRA provides information on legislation, regulations, procedures, approved products, approved companies, sanctions, recalls, public health warnings 8. Interaction with civil society 9. Intersectoral collaboration

Examples/criteria to review statutory basis • Drug legislation exists • NRA established and empowered in law • QC lab established and empowered in law • inspectorate empowered to access facilities, documentation, collect samples • legal provisions for marketing authorisations • legal provisions for control of importation • legal provisions for control of exportation • legal provisions for control of manufacturing • legal provisions for control of distribution (wholesale and retail) • legal provisions for monitoring safety of marketed products • legal provisions for monitoring quality of marketed products • legal provisions for control of promotion and advertising • legal provisions for control of clinical trials • legal provisions for appropriate sanctions • legal provisions requiring NRA to ensure confidentiality of data, and to be transparent and accountable • legal framework to identify and sanction illegal products and activities • regulations based on legislation issued for all areas, and kept up to date

Review findings can be summarized in graphical format % i m p l e m e n t e d NRA Key Functions 38 PAR Seminar 1 October 2003 WHO - HTP

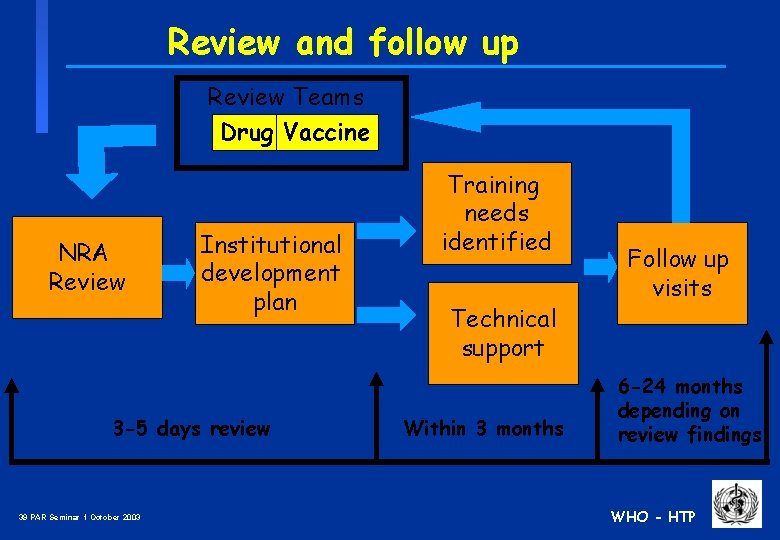
Review and follow up Review Teams Drug Vaccine NRA Review Institutional development plan 3 -5 days review 39 PAR Seminar 1 October 2003 Training needs identified Technical support Within 3 months Follow up visits 6 -24 months depending on review findings WHO - HTP

Outcome of a review visit • • opportunity for NRA staff to seat and look at own situation agreed document that highlights strengths and weaknesses and indicates priorities for action direct technical support and training document can be used to apply for additional support from other sources 40 PAR Seminar 1 October 2003 WHO - HTP

Achievements (1): reviews AFR Algeria Nigeria Zimbabwe Senegal South Africa Uganda AMR Argentina Bolivia Cuba Chile EMR Oman Saudi Arabia Syrian AR Egypt Iran Pakistan Tunisia Morocco EUR Hungary Kazakhstan Rep Moldova Turkmenistan Bulgaria Poland Romania Sweden Turkey Ukraine Uzbekistan Russia Joint Drug & Vaccine assessments 41 PAR Seminar 1 October 2003 SEAR Myanmar DPR Korea India Sri Lanka Thailand Indonesia Nepal WPR Philippines China Viet Nam Lao PDR Cambodia Malaysia Australia WHO - HTP

Achievements (2): synergy Drug/vaccine teams synergy benefits WHO work: n learning from each other (data collection tools, GTN) n same message to countries n harmonized technical support tools 42 PAR Seminar 1 October 2003 WHO - HTP

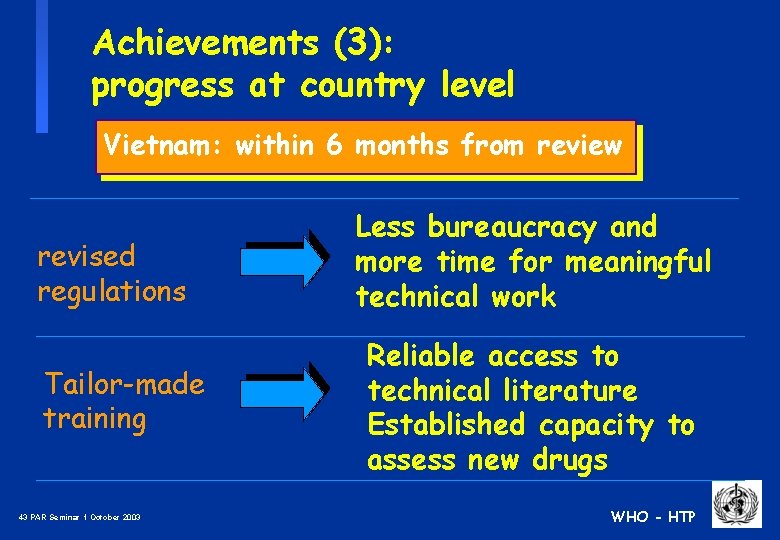
Achievements (3): progress at country level Vietnam: within 6 months from review revised regulations Tailor-made training 43 PAR Seminar 1 October 2003 Less bureaucracy and more time for meaningful technical work Reliable access to technical literature Established capacity to assess new drugs WHO - HTP

Achievements (4): progress at country level Tunisia: within 6 months from review Weak links with health system Survey to assess prescribers’ perception of regulatory work Inadequate drug information Ad hoc training and expertise to enable issuance of drug information by end 2002 for all drugs 44 PAR Seminar 1 October 2003 WHO - HTP

Thank you 45 PAR Seminar 1 October 2003 WHO - HTP
 California cpa regulatory review course
California cpa regulatory review course What do you think was askia’s greatest accomplishment?
What do you think was askia’s greatest accomplishment? Hebrews 12:12-13 the message
Hebrews 12:12-13 the message How did cardinal richelieu strengthen the french monarchy
How did cardinal richelieu strengthen the french monarchy Sustained synoynm
Sustained synoynm Channel capacity planning
Channel capacity planning Chase production method
Chase production method Assessing risk in sport regulatory bodies
Assessing risk in sport regulatory bodies Dispositional framework vs regulatory framework
Dispositional framework vs regulatory framework The purpose of traffic signs are
The purpose of traffic signs are Regulatory framework for financial services in india
Regulatory framework for financial services in india Chapter 22 regulatory and advisory agencies
Chapter 22 regulatory and advisory agencies Dea number verification
Dea number verification Infection control principles and practices milady
Infection control principles and practices milady Functions of sebi
Functions of sebi Regulatory institutions in indian financial system
Regulatory institutions in indian financial system Regulatory change management process
Regulatory change management process Duties of irda
Duties of irda Managing diversity and regulatory challenges
Managing diversity and regulatory challenges Regulatory sign color
Regulatory sign color Regulatory capital vs economic capital
Regulatory capital vs economic capital Regulatory capital vs economic capital
Regulatory capital vs economic capital Regulatory w automatyce
Regulatory w automatyce Gene regulatory network
Gene regulatory network State nuclear regulatory inspectorate of ukraine
State nuclear regulatory inspectorate of ukraine Vertical rectangles tell drivers:
Vertical rectangles tell drivers: Gene regulatory network
Gene regulatory network Regulatory reform fire safety order 2005
Regulatory reform fire safety order 2005 Film regulatory bodies
Film regulatory bodies Nespojité regulátory
Nespojité regulátory Minneapolis regulatory services
Minneapolis regulatory services Regulatory affairs kpi
Regulatory affairs kpi Post approval regulatory affairs
Post approval regulatory affairs Biologycorner.com
Biologycorner.com Fluorescent optic yellow road sign
Fluorescent optic yellow road sign Contoh kebijakan regulatif
Contoh kebijakan regulatif Regulatory framework of accounting
Regulatory framework of accounting Warehousing development and regulatory authority
Warehousing development and regulatory authority Regulatory creep
Regulatory creep The regulatory reform (fire safety) order 2005 summary
The regulatory reform (fire safety) order 2005 summary Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress National institute for food and drug surveillance
National institute for food and drug surveillance Regulatory readiness checklist
Regulatory readiness checklist Department of licensing and regulatory affairs
Department of licensing and regulatory affairs Missile launcher control system
Missile launcher control system Ingegneria sicurezza antincendio
Ingegneria sicurezza antincendio