Regulations August 2017 q Final 2018 MACRA regulations









- Slides: 12

Regulations – August, 2017 q Final 2018 MACRA regulations published q Awaiting CMS final rule on the 2018 Inpatient Prospective Payment System Published q 2018 Quality Payment Program Proposed Rule (comments – Aug 21, 2017) q 2018 Physician Fee Schedule Proposed Rule (comments – Sept 11, 2017) q 2018 Outpatient Prospective Payment System Proposed Rule (comments – Sept 11, 2017)

Possible comment areas - QPP q HL 7 Comment Area 1 - Possible Naming of FHIR-based Standards in Final Rule q “… we seek comment for future rulemaking regarding the potential shift to seeking alternatives which might fully replace the QRDA III format in the QPP in future program years…. ” q Comments: q HL 7 strongly believes that the FHIR-based standards and resources developed for quality reporting have reach a level of maturity and readiness in the industry sufficient for CMS to consider naming and incorporating in the final QPP regulation the following elements: q Use the FHIR Measure Report Resource for aggregate reporting to match current requirements (compatible and fully aligned with the intent of QRDA Category III). FHIR can support API-level submission as compared with submitting a file as required with QRDA. q Establish a transition period of 12 months during which both the current QRDA III and the FHIR Measure Report Resource will be acceptable for use. q If CMS wishes to expand to patient-level reporting, we recommend the individual-level report in the FHIR Measure Report Resource and the QI Core Profile to capture and represent quality information, and as based on the US Core profile to define measure content. We strongly endorse the use of US Core at a minimum as a step in the right direction for such individual-level reporting. q For patient-level reporting, it will be important to ensure that if both sets of standards are allowed to be used during a transition period, that both sets of standards produce consistent results for the same measures. This should be mitigated by the fact that the QDM will be mapped to QI Core and referenced in the FHIR Clinical Reasoning module.

Possible comment areas - QPP q Comments (cont. ): q Support both CQL-based HQMF measures using the QDM data model and FHIR-based measures using the Clinical Reasoning Module and QI Core. Either measure structure can be reported using individual-level or aggregate QRDA or FHIR reporting standards. q By naming these elements in the final QPP regulation, CMS will provide the industry with a roadmap for the future of quality reporting standards. This will also help fast-track the final refinement, adoption and use of these standards

Possible comment areas - QPP q HL 7 Comment Area 2: End-to-end electronic submission q NPRM notation: seeking comment on the use of health IT in quality measurement and how HHS can encourage the use of certified EHR technology in quality measurement as established in the statute. What other incentives within this category for reporting in an endto-end manner could be leveraged to incentivize more clinicians to report electronically? What format should these incentives take? For example, should clinicians who report all of their quality performance category data in an end-to-end manner receive additional bonus points than those who report only partial electronic data? Are there other ways that HHS should incentivize providers to report electronic quality data beyond what is currently employed? We welcome public comment on these questions. q HL 7 Comments: q The end-to-end bonus is not related to the use of standards. We recommend CMS consider modifying the bonus to encourage the use of standardized formats. q Use of established standards can enable a more streamlined submission process, perhaps a rolling submission rather than manual data entry for a reporting period done once per year. The FHIR-based submission capability aligns more closely with API submission.

Possible comment areas - QPP HL 7 Comment Area 3: Quality reporting submission mechanisms q q NPRM provides for different types of submission mechanisms q Question: Are this OK? Should there be changes in the list? Are there additions to the list? HL 7 Comments: q “Thus, we are proposing to revise § 414. 1390 to add a new paragraph (b) that requires all MIPS eligible clinicians and groups that submit data and inf --- ways to attest to accuracy of the content…. . ” q We want to note that QRDA or FHIR reporting standards allow entry of attestation that data are accurate and complete

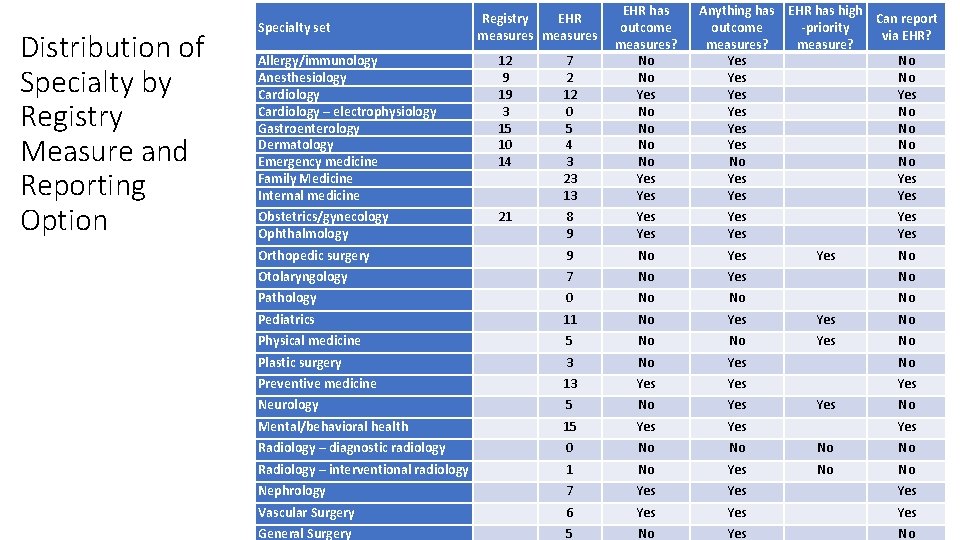
Possible comment areas - QPP HL 7 Comment Area 3: Quality reporting submission mechanisms (cont. ) q HL 7 Comments: q With the specialty measure sets that CMS is proposing, only 11 of the 35 specialties are able to report via standardized formats. (The rest are required to use e. g. claims because CMS has not designated any "high priority" e. CQMs as being part of their specialty measure set. ) q HL 7 would recommend that CMS considers modifying the specialty sets or creating new measures so that more specialists can gain the benefits which come from using standardized electronic reporting. q The majority of health care specialties, which represent a large proportion of patient in health care, are not being given this opportunity to report using these electronic standards q See table in next slide

Distribution of Specialty by Registry Measure and Reporting Option Allergy/immunology Anesthesiology Cardiology – electrophysiology Gastroenterology Dermatology Emergency medicine Family Medicine Internal medicine 12 9 19 3 15 10 14 7 2 12 0 5 4 3 23 13 EHR has outcome measures? No No Yes Obstetrics/gynecology Ophthalmology 21 8 9 Yes Yes Orthopedic surgery 9 No Yes Otolaryngology 7 No Yes No Pathology 0 No No No Pediatrics 11 No Yes No Physical medicine 5 No No Yes No Plastic surgery 3 No Yes No Preventive medicine 13 Yes Yes Neurology 5 No Yes Mental/behavioral health 15 Yes Radiology – diagnostic radiology 0 No No Radiology – interventional radiology 1 No Yes No No Nephrology 7 Yes Yes Vascular Surgery 6 Yes Yes General Surgery 5 No Yes No Specialty set Registry EHR measures Anything has EHR has high Can report outcome -priority via EHR? measures? measure? Yes No Yes No No No Yes Yes No No Yes

Possible comment areas - QPP HL 7 Comment Area 4: The costs and benefits of using standards for reporting q Request: HL 7 comment(s) on the benefits and cost-savings of utilizing electronic standards (in general) and the FHIR-based standards being recommended for inclusion in the final QPP rule q HL 7 comments: q Use of FHIR-based standards streamlines submission and aligns with the use of APIs q Using standards in individual-level and aggregate reporting, increases the likelihood for submission of accurate and precise data q Use of standards consistent with those used for routine information sharing (interoperability) decreases the complexity of reporting. Quality reporting involves patient data and non-standard specifications for those data increase costs and complexity. Ideally, the number of patient data formats should be reduced. A non-standard approach has the risk of creating a new format. Use the FHIR maturity model to assess the maturity of potential new approaches. Use of FHIR-based standards aligns with a standard data format and encourages a common standard for all reporting. The FHIR maturity model encourages development and use of resources and profiles that address information present in real-world settings. q QRDA or FHIR reporting standards could allow entry of provider attestation that data are accurate and complete. Conformance testing with standards helps assure the formatting of submitted data is correct.

Regulations – August, 2017 q Final 2018 MACRA regulations published q Awaiting CMS final rule on the 2018 Inpatient Prospective Payment System Published q 2018 Quality Payment Program Proposed Rule (comments – Aug 21, 2017) q 2018 Physician Fee Schedule Proposed Rule (comments – Sept 11, 2017) q 2018 Outpatient Prospective Payment System Proposed Rule (comments – Sept 11, 2017)

Final CMS 2018 IPPS Rule q Pre-publication (2, 400+ pages) published last week; formal publication in the Federal Register will be August 14, 2017 q One of the core annual CMS rules related to the administration of the Medicare and Medicaid programs q Covers acute and long-term care hospital payment systems and rates for 2018, quality reporting requirements; Medicare/Medicaid EHR Incentive Program (Meaningful Use); cost reporting and provider requirements; and other topics q Two areas to review: q Meaningful Use Program changes q Quality reporting requirements

Final CMS 2018 IPPS Rule – Meaningful Use q q q Significant changes to the MU Stage 3 program – 2018 reporting year Confirmed proposed 90 -day reporting period for 2018, which apply to all new and returning EPs, EHs and CAHs Provides flexibility to use 2014 -Edition certified EHRs or 2015 -Edition certified EHRs. This flexibility means that reporting MU Stage 3 metrics during 2018 that require the use of 2015 Edition certified EHRs is, ultimately, optional. This is especially important when considering such new MU 3 functionality and requirements as the use of APIs for consumer access to health information. Bottom-line: q Modified Stage 2 was formerly 2015 -2017 and is now 2015 -2018, ending in 2018. During 2018, EPs, EHs and CAHs will be required to meet the Modified Stage 2 requirements using EHRs that have been certified with 2014 -Edition certification criteria, and report those metrics for a 90 -day period. q Stage 3 was optional in 2017 is now optional also for 2018, and fully required in 2019. Providers will be able to report MU Stage 3 measures optionally in 2018 with EHRs that have been able to certify for the 2015 -Edition certification criteria. q Industry will have one more year to get 2015 -Edition EHRs certified (2018) to start fully reporting MU 3 metrics in 2019 (only MU 3 metrics, full year reporting)

Final CMS 2018 IPPS Rule – Quality Reporting q Require use of QRDA Cat I for reporting individual Medicare FFS beneficiary e. CQM data. Same standard required for submitting core clinical data elements to CMS q Hospitals to report on at least 4 of the 15 available e. CQMs (instead of 8) and submit data for 1 self-selected calendar quarter, instead of a full calendar year of data q Several comments regarding concerns about the considerable burden required to map the necessary data elements from the EHR to the appropriate QRDA file format given that some vendors are not properly equipped to collect and transmit such data through the CMS Quality. Net Secure Portal. There also some limitations of the Portal (inability to accept QRDA I files over a certain size (5 MB). q Issue with use of 2015 -Edition certified EHRs vs 2014 -Edition certified EHRs. Number of 2015 -Edition certified EHRs as of June 30 th much lower than ONC estimated. While ONC estimate that upward of 75% of hospital EHRs will be certified for 2015 -Edition criteria by end of CY 2017, there is little or no time to do the transition to new version before 2018 starts. Thus, CMS is allowing, in the final rule, flexibility for hospitals to use 2014 -Edition certified EHRs, 2015 -Edition certified EHRs, or combination of both in 2018 reporting period. They note they have allowed such flexibility in 2017, and are not aware of any issues in QRDA I file creation/submission under 2014 Edition or 2015 -Edition EHRs q In the FY 2016 final rule CMS removed the QRDA III as an option for reporting e. CQMs under the MU program and required QRDA I. States continue to have the option (subject to prior CMS approval) of allowing or requiring QRDA III for CQM reporting q No mentioning of HQMF, FHIR, CQL, CQF, or other quality reporting standards/artifact