Radiation definitions of activity dose and dose rates







- Slides: 24

Radiation – definitions of activity, dose, and dose rates presentation for Physics 125 This is optional material.

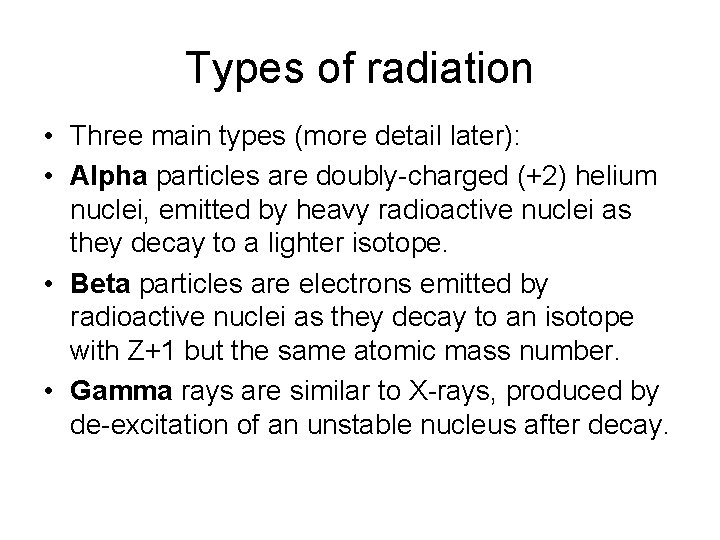
Types of radiation • Three main types (more detail later): • Alpha particles are doubly-charged (+2) helium nuclei, emitted by heavy radioactive nuclei as they decay to a lighter isotope. • Beta particles are electrons emitted by radioactive nuclei as they decay to an isotope with Z+1 but the same atomic mass number. • Gamma rays are similar to X-rays, produced by de-excitation of an unstable nucleus after decay.

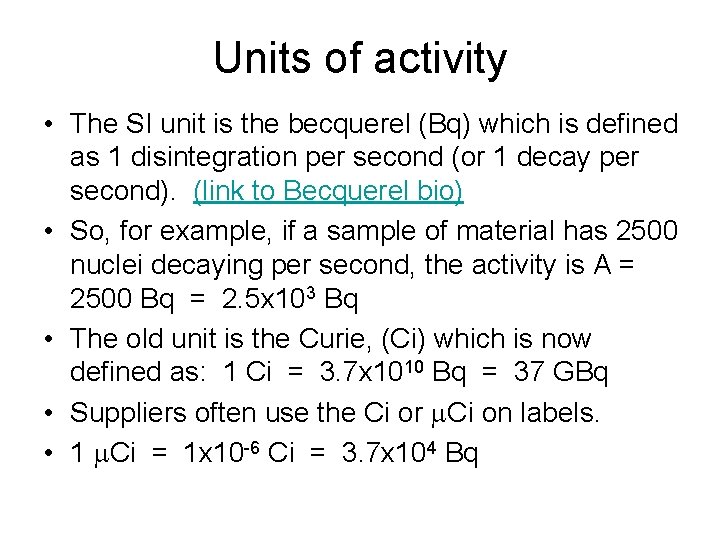
Units of activity • The SI unit is the becquerel (Bq) which is defined as 1 disintegration per second (or 1 decay per second). (link to Becquerel bio) • So, for example, if a sample of material has 2500 nuclei decaying per second, the activity is A = 2500 Bq = 2. 5 x 103 Bq • The old unit is the Curie, (Ci) which is now defined as: 1 Ci = 3. 7 x 1010 Bq = 37 GBq • Suppliers often use the Ci or m. Ci on labels. • 1 m. Ci = 1 x 10 -6 Ci = 3. 7 x 104 Bq

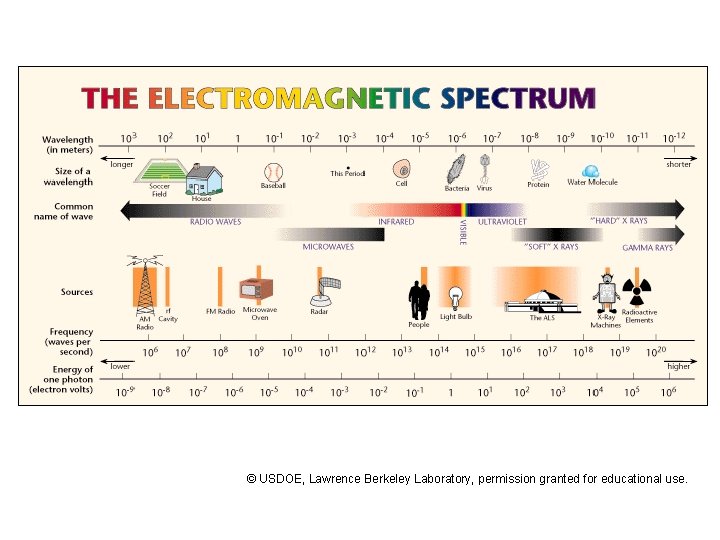
X-rays and g rays • X-rays and gamma rays are at one end of the electromagnetic spectrum, which includes radio waves, microwaves, infrared light, visible light, and ultraviolet light. • Electromagnetic waves are characterized by frequency, wavelength, and speed. • fl = c where c = 299792458 m/s is the speed of light in vacuum, c = 3 x 108 m/s for common use. • For X-rays and gamma rays, v ~ c in most materials (the index of refraction is about 1).

The photon concept • X-rays and gamma rays have higher frequency than ultraviolet light, and their wavelength and frequency are not relevant for many situations. • Electromagnetic waves also exhibit particle-like properties (Max Planck and Albert Einstein). • Photon energy is E = hf where h is Planck’s constant, h = 6. 63 x 10 -34 J. s, and f is frequency. • Most X-rays and gamma rays travel in a straight line until they are absorbed by an atom in a process called the photoelectric effect.

© USDOE, Lawrence Berkeley Laboratory, permission granted for educational use.

© USDOE, Lawrence Berkeley Laboratory, permission granted for educational use. Wavelength, meters Frequency, Hz Photon energy, e. V

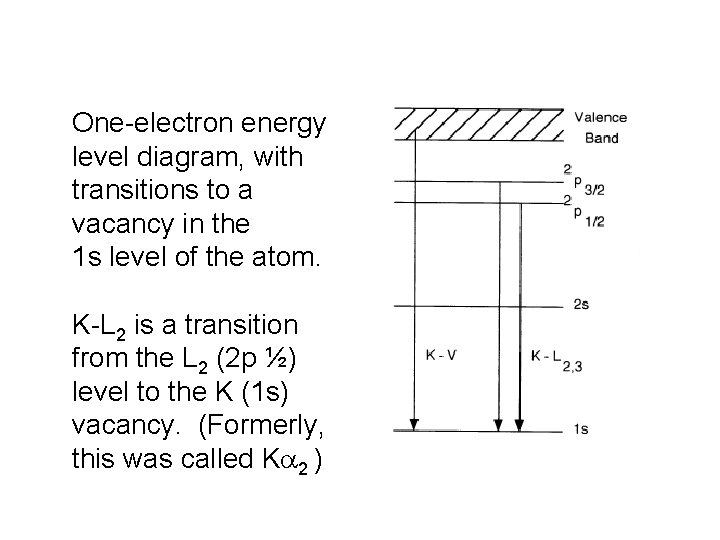
Sources of X-rays • X-rays are usually produced by atomic transitions in X-ray tubes or very hot plasma. • X-rays can also be produced by accelerated electrons: “braking radiation” (bremsstrahlung) in X-ray tubes, or in the strong magnetic fields in synchrotrons and particle accelerators. • X-rays can also be produced from extremely hot plasmas (nuclear explosions and stars). • X-ray emission from an atom results when there is a vacancy in an inner shell and another electron can “fall” into the lower energy level.

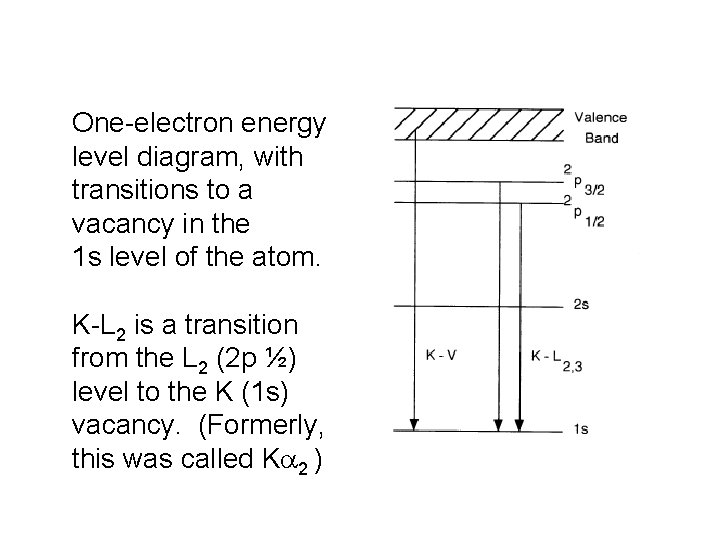
One-electron energy level diagram, with transitions to a vacancy in the 1 s level of the atom. K-L 2 is a transition from the L 2 (2 p ½) level to the K (1 s) vacancy. (Formerly, this was called Ka 2 )

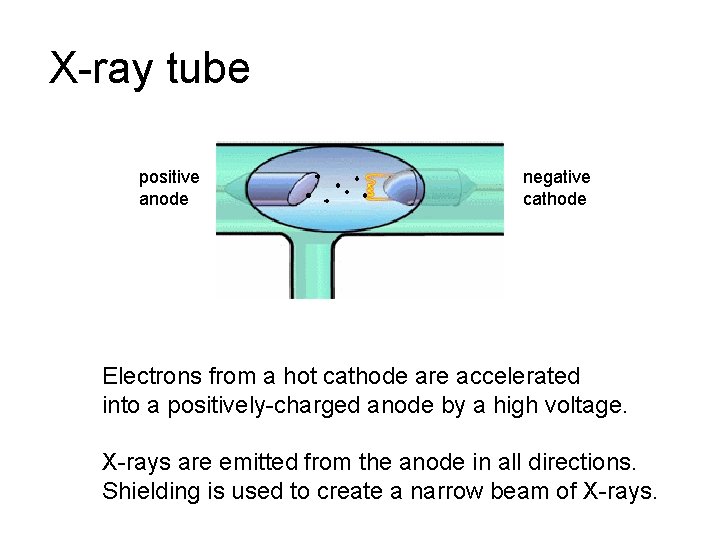
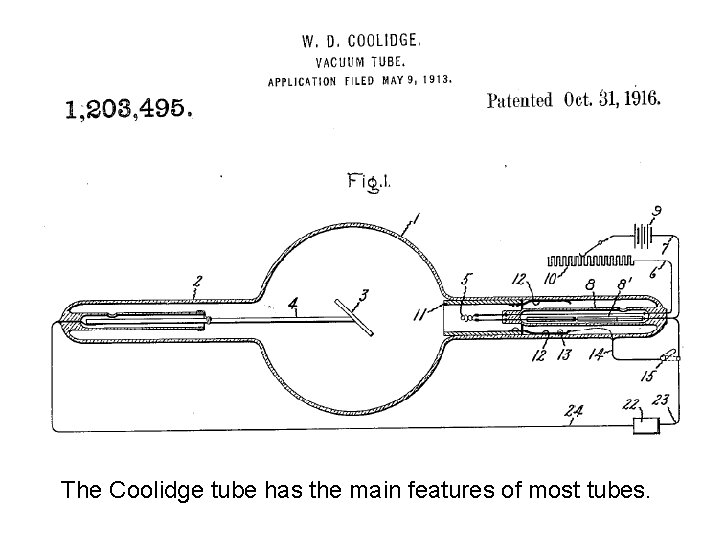
X-ray tube positive anode negative cathode Electrons from a hot cathode are accelerated into a positively-charged anode by a high voltage. X-rays are emitted from the anode in all directions. Shielding is used to create a narrow beam of X-rays.

Many types of X-ray tube have been developed since the early days.

The Coolidge tube has the main features of most tubes.

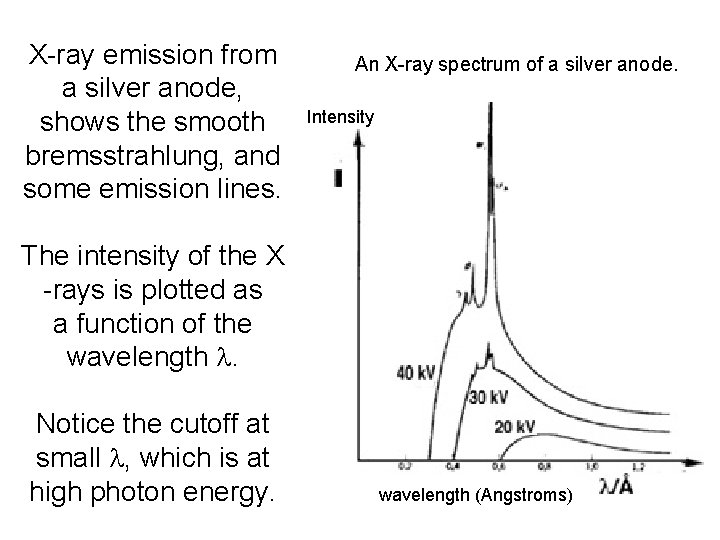
X-ray emission from a silver anode, shows the smooth bremsstrahlung, and some emission lines. An X-ray spectrum of a silver anode. Intensity The intensity of the X -rays is plotted as a function of the wavelength l. Notice the cutoff at small l, which is at high photon energy. wavelength (Angstroms)

One-electron energy level diagram, with transitions to a vacancy in the 1 s level of the atom. K-L 2 is a transition from the L 2 (2 p ½) level to the K (1 s) vacancy. (Formerly, this was called Ka 2 )

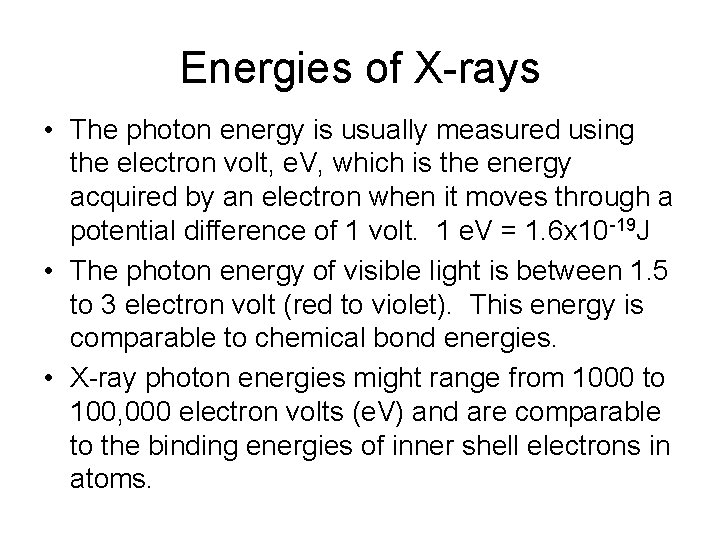
Energies of X-rays • The photon energy is usually measured using the electron volt, e. V, which is the energy acquired by an electron when it moves through a potential difference of 1 volt. 1 e. V = 1. 6 x 10 -19 J • The photon energy of visible light is between 1. 5 to 3 electron volt (red to violet). This energy is comparable to chemical bond energies. • X-ray photon energies might range from 1000 to 100, 000 electron volts (e. V) and are comparable to the binding energies of inner shell electrons in atoms.

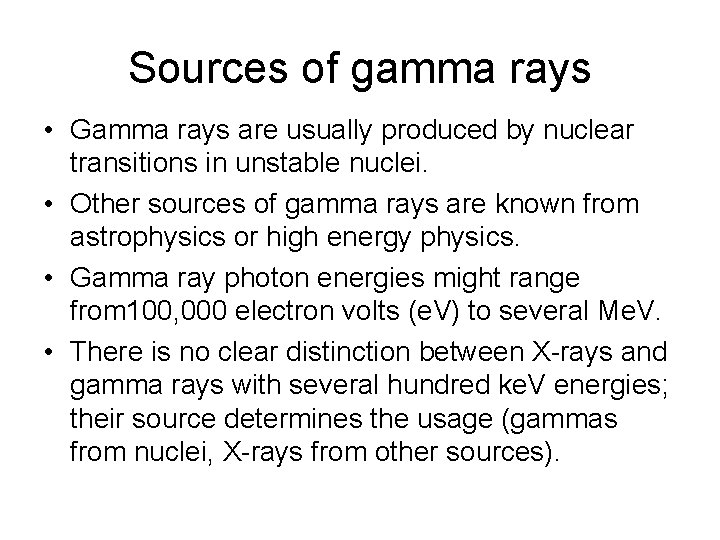
Sources of gamma rays • Gamma rays are usually produced by nuclear transitions in unstable nuclei. • Other sources of gamma rays are known from astrophysics or high energy physics. • Gamma ray photon energies might range from 100, 000 electron volts (e. V) to several Me. V. • There is no clear distinction between X-rays and gamma rays with several hundred ke. V energies; their source determines the usage (gammas from nuclei, X-rays from other sources).

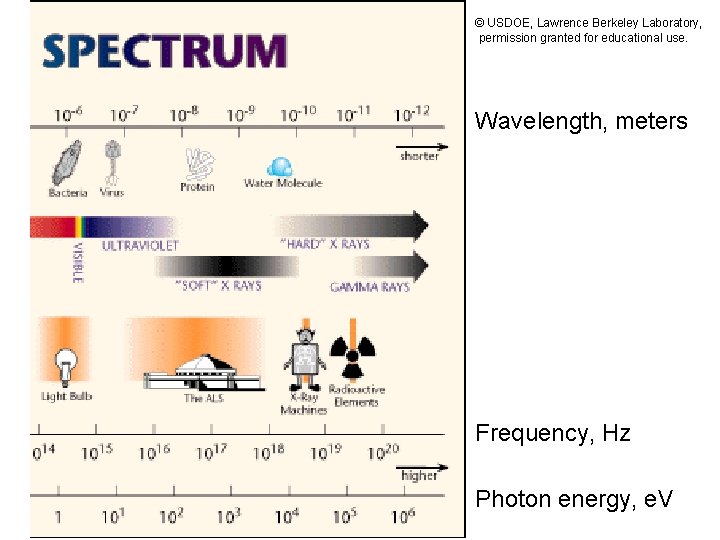
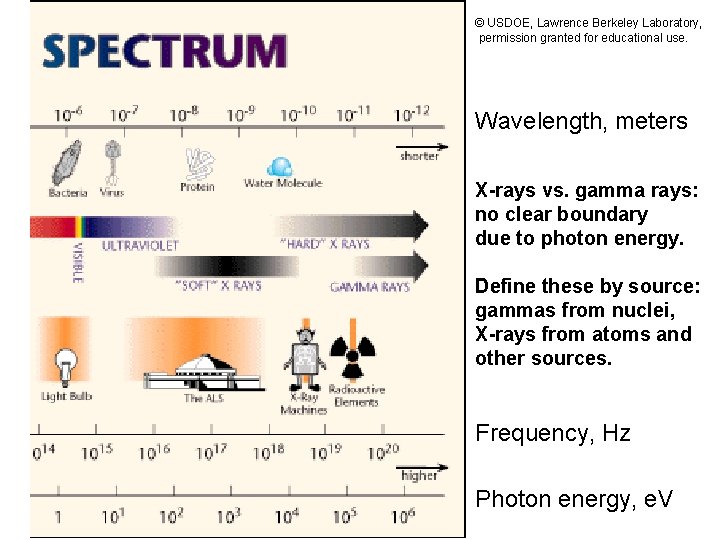
© USDOE, Lawrence Berkeley Laboratory, permission granted for educational use. Wavelength, meters X-rays vs. gamma rays: no clear boundary due to photon energy. Define these by source: gammas from nuclei, X-rays from atoms and other sources. Frequency, Hz Photon energy, e. V

Radiation dose, dose rate • When radiation (electromagnetic or particles) is absorbed by any material, energy is absorbed by the material (photon energy or kinetic energy). • The physical unit of absorbed radiation dose is the gray (Gy), equal to 1 J per kg of mass. • Dose rate is the rate of dosage, the dose per unit time interval, for example, 10 m. Gy/h. • This is a physical measurement, and does not correctly quantify the long-term biological effect.

Equivalent dose • Different types of radiation (alpha, beta, gamma, neutrons) have different efficiencies for causing long-term biological effects, for the same physical dose into living material. • The unit of ‘equivalent dose’ is the sievert (Sv), obtained by multiplying the physical dose by a weighting factor (w. R) which reflects the biological effectiveness of different radiations. • Dose in Sv is the dose in Gy multiplied by w. R.

Weighting factor (w. R) • The weighting factor (w. R) is 1 for beta particles. • The weighting factor (w. R) is also 1 for X-rays and gamma rays. • The weighting factor (w. R) is 20 for alpha particles and 5 -20 for neutrons, depending on energy. • Some beta particles may not be much hazard because they have low energy and will not penetrate the skin (for example, from tritium). • Alpha particles also will not penetrate skin.

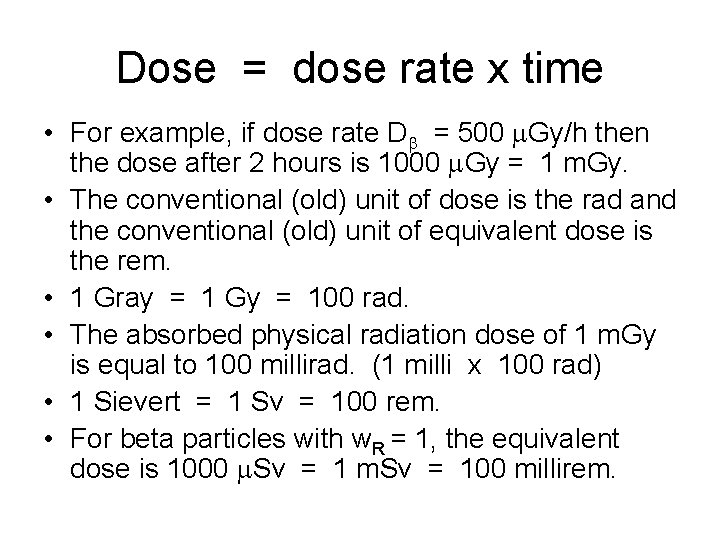
Dose = dose rate x time • For example, if dose rate Db = 500 m. Gy/h then the dose after 2 hours is 1000 m. Gy = 1 m. Gy. • The conventional (old) unit of dose is the rad and the conventional (old) unit of equivalent dose is the rem. • 1 Gray = 1 Gy = 100 rad. • The absorbed physical radiation dose of 1 m. Gy is equal to 100 millirad. (1 milli x 100 rad) • 1 Sievert = 1 Sv = 100 rem. • For beta particles with w. R = 1, the equivalent dose is 1000 m. Sv = 100 millirem.

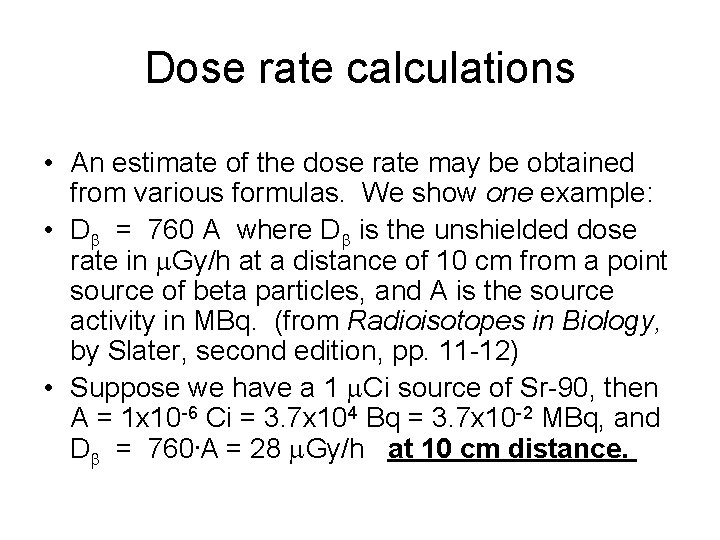
Dose rate calculations • An estimate of the dose rate may be obtained from various formulas. We show one example: • Db = 760 A where Db is the unshielded dose rate in m. Gy/h at a distance of 10 cm from a point source of beta particles, and A is the source activity in MBq. (from Radioisotopes in Biology, by Slater, second edition, pp. 11 -12) • Suppose we have a 1 m. Ci source of Sr-90, then A = 1 x 10 -6 Ci = 3. 7 x 104 Bq = 3. 7 x 10 -2 MBq, and Db = 760. A = 28 m. Gy/h at 10 cm distance.

Dose rate calculations, misc. • Different formulas are used for different types of radiation, such as a, b, X-rays and gamma rays. • These formulas also require an understanding of the distance dependence of radiation (i. e. , the inverse square law), and the effects of shielding. • Why are these calculations important? • Radiation dose causes biological effects.

Biological effects of radiation on humans: the basis of legislation Tissue level effects: whole body, local, or in utero Whole body effects (clinical symptoms) are seen above 1 Gy = 100 rad, but this only occurs in serious accidents or nuclear warfare. Local effects: radiation burns have a threshold around 3 Gy. Above 10 Gy, serious burns cause ulceration and blistering. Beta sources (esp. in liquid forms) can be a cause of lab accidents that cause these serious burns.
 Unit ratio
Unit ratio Equivalent ratios definition
Equivalent ratios definition Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Radiation dose limits for workers and public pdf
Radiation dose limits for workers and public pdf Radiation dose limits
Radiation dose limits Radiation dose limits
Radiation dose limits Radiation dose limits chart
Radiation dose limits chart Debye huckel limiting law
Debye huckel limiting law Reactants, products, and leftovers
Reactants, products, and leftovers Aon network examples
Aon network examples Activity 1 introductory activity
Activity 1 introductory activity Activity 2 finding the sequence
Activity 2 finding the sequence 3
3 Activity 1 activity 2
Activity 1 activity 2 Verbal irony definition
Verbal irony definition The problem of concept drift: definitions and related work
The problem of concept drift: definitions and related work The correct shutoff procedure for an oxyacetylene torch is:
The correct shutoff procedure for an oxyacetylene torch is: Cheek cut rafter
Cheek cut rafter Material properties definitions
Material properties definitions What does incessant mean in hatchet
What does incessant mean in hatchet 8 news values and definitions
8 news values and definitions Comparison and contrast definition
Comparison and contrast definition Cscmp supply chain management definitions and glossary
Cscmp supply chain management definitions and glossary Undefined terms definition
Undefined terms definition