Quantum Theory n n n n Schroedingers Cat






- Slides: 19

Quantum Theory n n n n Schroedinger’s Cat Place a cat in a box Also place a radioactive isotope and a vial of poison The isotope decays once per hour If the particle triggers a Geiger counter, the cat dies If the Geiger counter is not triggered, the cat lives Seal the box and wait an hour What happened to the cat?

Electromagnetic Spectrum n The speed of light© is 3. 00 x 108 m/s


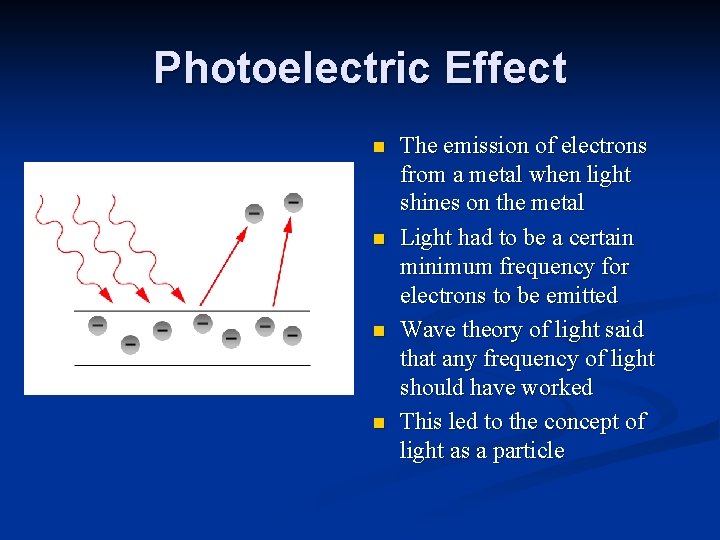
Photoelectric Effect n n The emission of electrons from a metal when light shines on the metal Light had to be a certain minimum frequency for electrons to be emitted Wave theory of light said that any frequency of light should have worked This led to the concept of light as a particle

Light as a particle n n n Max Planck (1900) Hot objects emit light and other forms of electromagnetic radiation, but not continuously, as expected Instead, it is emitted in small, specific amounts called quanta. E=hv H=6. 626 x 10 -34 J*s This number is called Planck’s constant


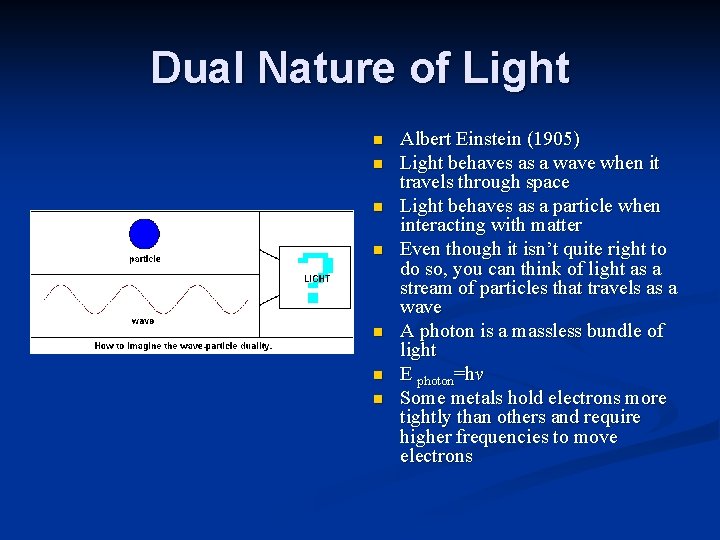
Dual Nature of Light n n n n Albert Einstein (1905) Light behaves as a wave when it travels through space Light behaves as a particle when interacting with matter Even though it isn’t quite right to do so, you can think of light as a stream of particles that travels as a wave A photon is a massless bundle of light E photon=hv Some metals hold electrons more tightly than others and require higher frequencies to move electrons

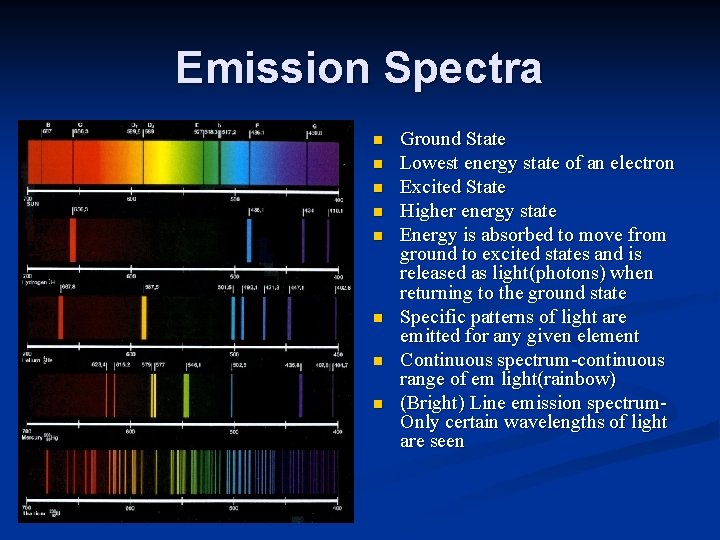
Emission Spectra n n n n Ground State Lowest energy state of an electron Excited State Higher energy state Energy is absorbed to move from ground to excited states and is released as light(photons) when returning to the ground state Specific patterns of light are emitted for any given element Continuous spectrum-continuous range of em light(rainbow) (Bright) Line emission spectrum. Only certain wavelengths of light are seen

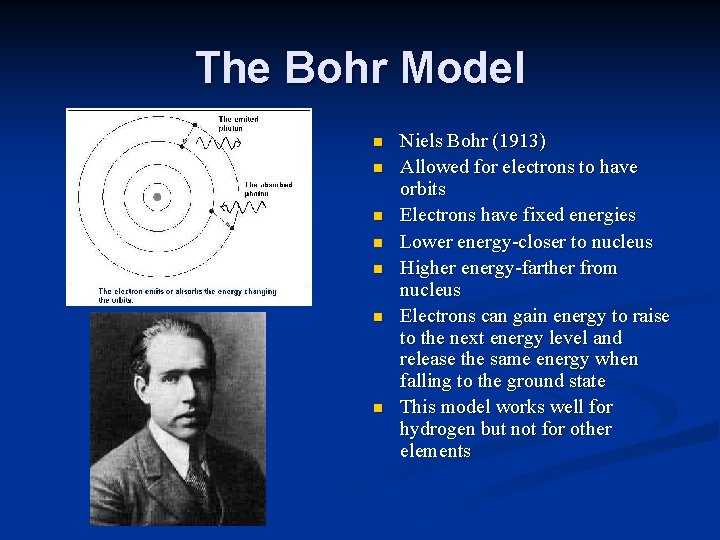
The Bohr Model n n n n Niels Bohr (1913) Allowed for electrons to have orbits Electrons have fixed energies Lower energy-closer to nucleus Higher energy-farther from nucleus Electrons can gain energy to raise to the next energy level and release the same energy when falling to the ground state This model works well for hydrogen but not for other elements

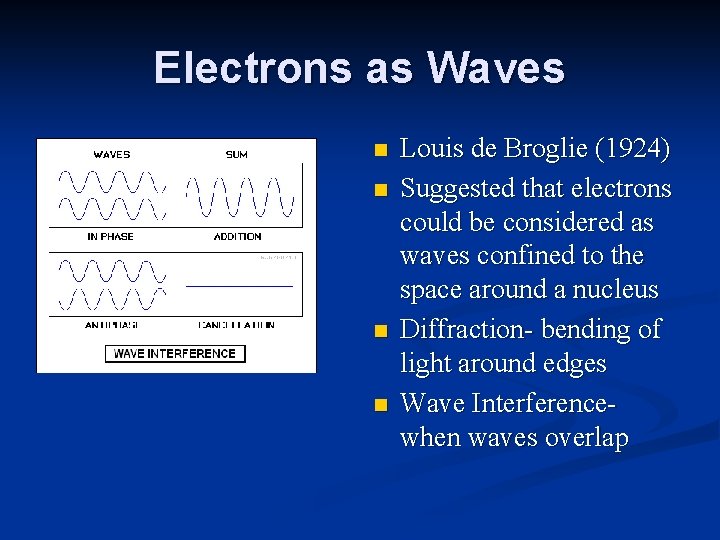
Electrons as Waves n n Louis de Broglie (1924) Suggested that electrons could be considered as waves confined to the space around a nucleus Diffraction- bending of light around edges Wave Interferencewhen waves overlap

Quantum Theory Werner Heisenberg (1927) n The Uncertainty Principle n One cannot simultaneously know the position and velocity of an electron n

Quantum Theory n n n Erwin Schroedinger (1926) Wave Equation- The quantization of an electron’s energies is an outcome Quantum Theorymathematically describes wave properties of electrons and other small particles

Atomic Orbitals and Quantum Numbersproperties of atomic orbitals and properties of electrons in orbitals n Like the address of an electron or a seat in a stadium or theater n

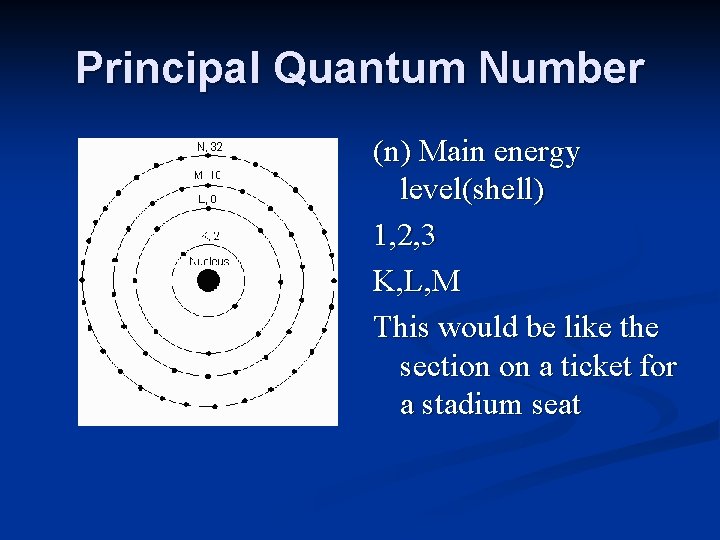
Principal Quantum Number (n) Main energy level(shell) 1, 2, 3 K, L, M This would be like the section on a ticket for a stadium seat

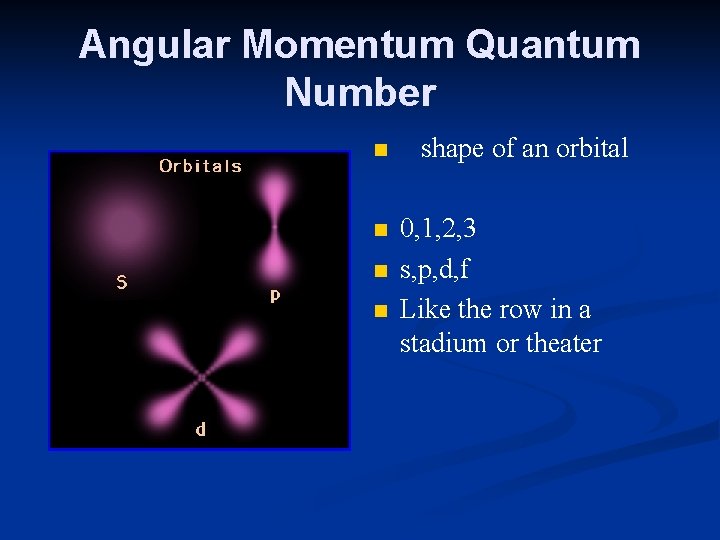
Angular Momentum Quantum Number n n shape of an orbital 0, 1, 2, 3 s, p, d, f Like the row in a stadium or theater

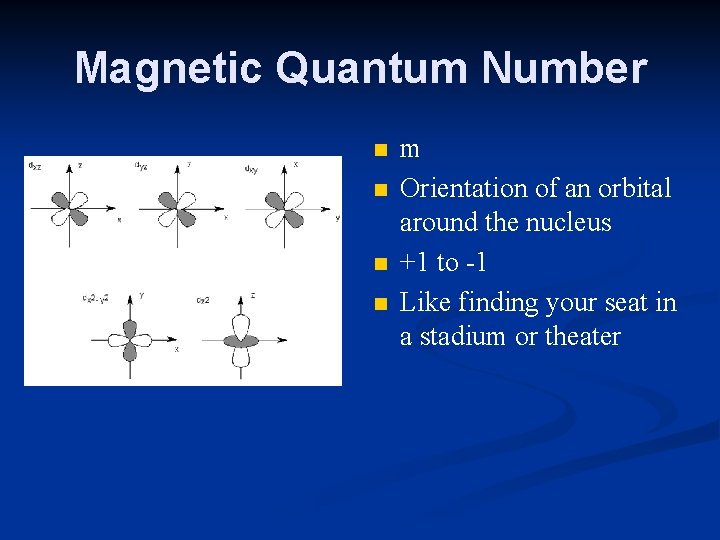
Magnetic Quantum Number n n m Orientation of an orbital around the nucleus +1 to -1 Like finding your seat in a stadium or theater

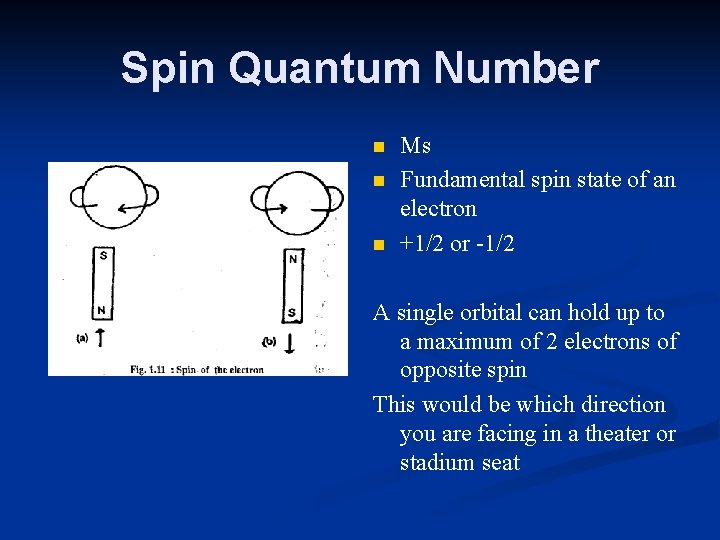
Spin Quantum Number n n n Ms Fundamental spin state of an electron +1/2 or -1/2 A single orbital can hold up to a maximum of 2 electrons of opposite spin This would be which direction you are facing in a theater or stadium seat


Some Quantum Theory Rules n n n Pauli Exclusion Principle- No two electrons have the same set of four quantum numbers. Or An orbital within a sublevel can contain up to 2 electrons of opposite spin Hund’s Rule- Each orbital within a sublevel receives an electron of positive spin before any can receive an electron of negative spin