QSARs and Inorganic Chemistry What is QSAR Quantitative




- Slides: 9

QSARs and Inorganic Chemistry • What is QSAR? • Quantitative Structure‐Activity Relationship • Way to quantitatively correlate structure to physical properties or biological activity • Can you correlate systematic changes in structure and/or composition to a measurable trend in properties? • Related to the physical‐organic chemistry concept of Hammett parameters • Hammett asked “How do electronic effects influence reaction equilibria, Keq? ” • Original studies used the dissociation of p‐substituted benzoic acids • As early example of a linear‐free energy relationship

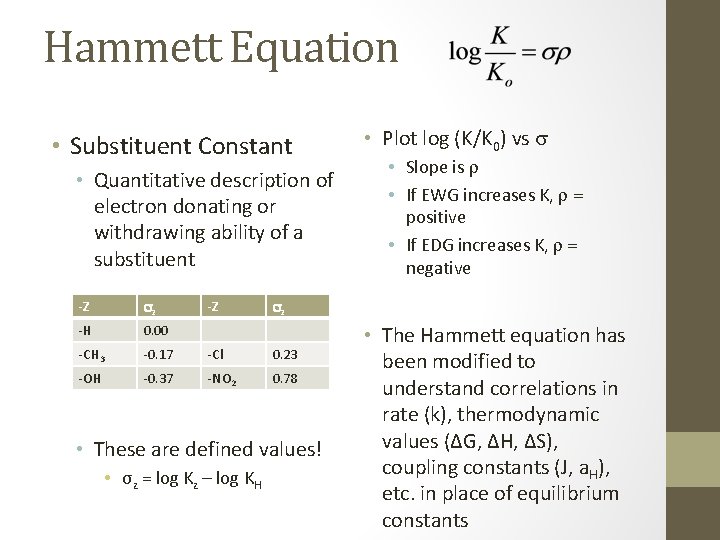
Hammett Equation • Substituent Constant • Quantitative description of electron donating or withdrawing ability of a substituent -Z sz ‐H 0. 00 ‐CH 3 ‐ 0. 17 ‐Cl 0. 23 ‐OH ‐ 0. 37 ‐NO 2 0. 78 -Z • Slope is r • If EWG increases K, r = positive • If EDG increases K, r = negative sz • These are defined values! • σz = log Kz – log KH • Plot log (K/K 0) vs s • The Hammett equation has been modified to understand correlations in rate (k), thermodynamic values (ΔG, ΔH, ΔS), coupling constants (J, a. H), etc. in place of equilibrium constants

Modification of σ • Substituent constants (σ) are not “one size fits all” • Formally, σ describes electronic effects seen in para‐ substituted benzoic acids • Includes both inductive and resonance effects • σ has been modified to separate out these two effects • These values are redefined as σR and σI , resonance and inductive respectively • Additional modifications to σ have been published • These include: • • σ. = radical intermediates σ‐= negatively charged intermediates σ+ = positively charged intermediates σm = meta substituted compounds…

How is this applied to inorganic chemistry? • Correlating structure and property relationships can give information regarding: • Mechanistic information • k/K changes with changing properties • Intermediates • Predictive power • Regular trends can be elucidated • Help guide future studies/synthetic efforts • Structural changes can be made to: • Ligands • Metal center

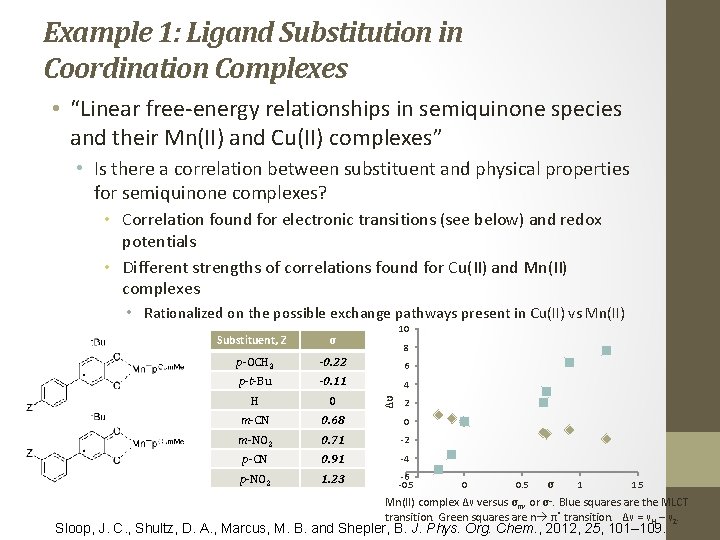
Example 1: Ligand Substitution in Coordination Complexes • “Linear free‐energy relationships in semiquinone species and their Mn(II) and Cu(II) complexes” • Is there a correlation between substituent and physical properties for semiquinone complexes? • Correlation found for electronic transitions (see below) and redox potentials • Different strengths of correlations found for Cu(II) and Mn(II) complexes • Rationalized on the possible exchange pathways present in Cu(II) vs Mn(II) 10 Substituent, Z σ p-OCH 3 -0. 22 6 p-t-Bu -0. 11 4 H 0 m-CN 0. 68 0 m-NO 2 0. 71 ‐ 2 p-CN 0. 91 ‐ 4 p-NO 2 1. 23 ‐ 6 ‐ 0. 5 Δυ 8 2 1 1. 5 σ − Mn(II) complex Δν versus σm, or σ. Blue squares are the MLCT transition. Green squares are n π* transition. Δν = νH – νZ. 0 0. 5 Sloop, J. C. , Shultz, D. A. , Marcus, M. B. and Shepler, B. J. Phys. Org. Chem. , 2012, 25, 101– 109.

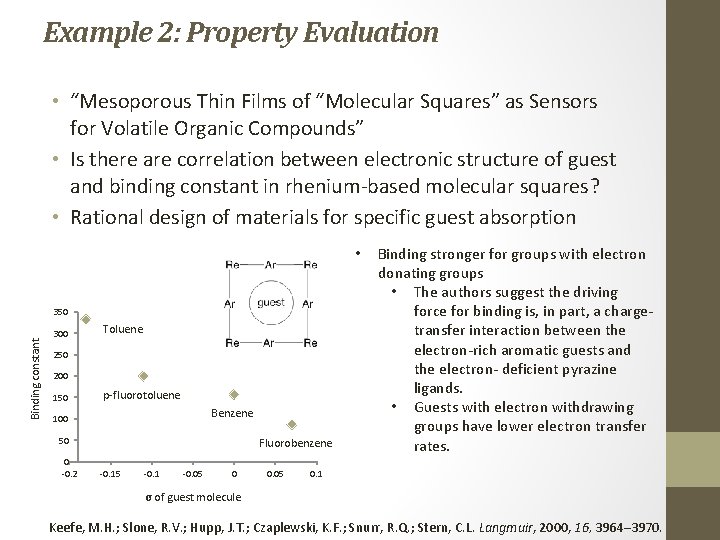
Example 2: Property Evaluation • “Mesoporous Thin Films of “Molecular Squares” as Sensors for Volatile Organic Compounds” • Is there are correlation between electronic structure of guest and binding constant in rhenium‐based molecular squares? • Rational design of materials for specific guest absorption • Binding constant 350 300 Toluene 250 200 150 p‐fluorotoluene Benzene 100 50 0 ‐ 0. 2 Fluorobenzene ‐ 0. 15 ‐ 0. 1 ‐ 0. 05 0 0. 05 Binding stronger for groups with electron donating groups • The authors suggest the driving force for binding is, in part, a charge‐ transfer interaction between the electron‐rich aromatic guests and the electron‐ deficient pyrazine ligands. • Guests with electron withdrawing groups have lower electron transfer rates. 0. 1 σ of guest molecule Keefe, M. H. ; Slone, R. V. ; Hupp, J. T. ; Czaplewski, K. F. ; Snurr, R. Q. ; Stern, C. L. Langmuir, 2000, 16, 3964– 3970.

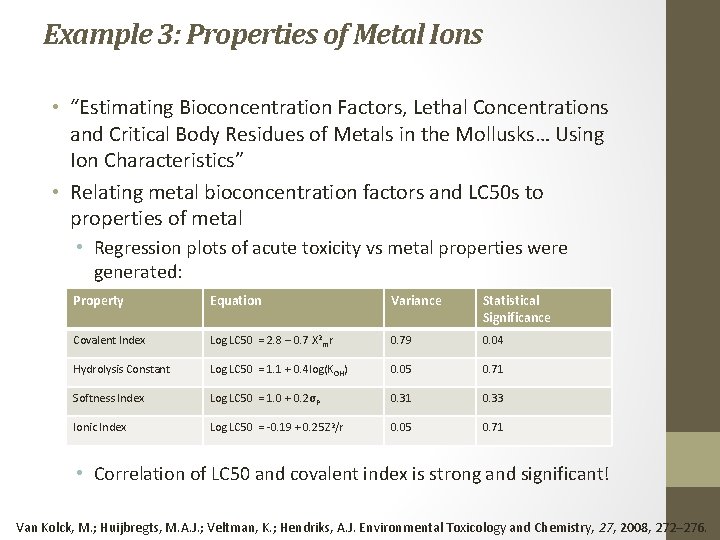
Example 3: Properties of Metal Ions • “Estimating Bioconcentration Factors, Lethal Concentrations and Critical Body Residues of Metals in the Mollusks… Using Ion Characteristics” • Relating metal bioconcentration factors and LC 50 s to properties of metal • Regression plots of acute toxicity vs metal properties were generated: Property Equation Variance Statistical Significance Covalent Index Log LC 50 = 2. 8 – 0. 7 Χ 2 mr 0. 79 0. 04 Hydrolysis Constant Log LC 50 = 1. 1 + 0. 4 log(K OH) 0. 05 0. 71 Softness Index Log LC 50 = 1. 0 + 0. 2σ P 0. 31 0. 33 Ionic Index Log LC 50 = ‐ 0. 19 + 0. 25 Z 2/r 0. 05 0. 71 • Correlation of LC 50 and covalent index is strong and significant! Van Kolck, M. ; Huijbregts, M. A. J. ; Veltman, K. ; Hendriks, A. J. Environmental Toxicology and Chemistry, 27, 2008, 272– 276.

Further Reading • T. H. Lowry, K. S. Richardson. Mechanism and Theory in Organic Chemistry, 2 nd ed. Harper Collins, 1987, pp 143 – 159. • Walker, J. Newman, M. C. , Enache M. Fundamental QSARs for Metal Ions. Taylor & Francis, Boca Raton, FL, 2012. • Journals that publish QSAR/SAR related research • http: //www. qsarworld. com/literature‐qsar‐journals. php • Review with values of σ for many organic and inorganic substituents • C. Hansch, A. Leo and R. W. Taft (1991). "A survey of Hammett substituent constants and resonance and field parameters". Chem. Rev. 91 (2): 165– 195. • http: //pubs. acs. org/doi/abs/10. 1021/cr 00002 a 004

Learning Outcomes • Define QSAR • Describe the Hammett equation including definitions of each variable • Give examples of how QSAR can be used to predict properties of inorganic systems