QAPI Implementation Phase 3 CMS Requirements of Participation














- Slides: 19

QAPI Implementation: Phase 3 CMS Requirements of Participation

CMS Requirements of Participation § 483. 75 Quality Assurance and Performance Improvement Phase 1 – November 28, 2016 • Disclosure of information • Sanctions Phase 2 – November 28, 2017 • Initial QAPI Plan must be provided to State Agency Surveyor at annual survey Phase 3 – November 28, 2019 • Implementation of the QAPI Program (including PIPs) • Addition of the (ICPO) Infection Control Prevention Officer • Governing body responsible for QAPI Program

Overview: § 483. 75 QAPI Regulations • Each LTC facility, including a facility that is part of a multiunit chain, must develop, implement, and maintain an effective, comprehensive, data-driven QAPI program that focuses on indicators of the outcomes of care and quality of life. • Maintain documentation and demonstrate evidence of its ongoing QAPI program • The 5 QAPI Program Elements: 1. Design and Scope 2. Governance and Leadership 3. Feedback, Data Systems, and Monitoring 4. Performance Improvement Projects (PIPs) 5. Systematic Analysis and Systemic Action

Overview: § 483. 75 QAPI Regulations • The QAPI program is sustained during transitions in leadership and staffing • The QAPI program is adequately resourced, including ensuring staff time, equipment, and technical training as needed • QAPI program identifies and prioritizes problems and opportunities • Corrective actions address gaps in systems, and are evaluated for effectiveness • Clear expectations are set around safety, quality, residents’ rights, choices, and respect. • The governing body and/or executive leadership is responsible and accountable for ensuring that: o An ongoing QAPI program: • Is maintained and addresses identified priorities • Is sustained during transitions in leadership and staffing • Is adequately resourced, including ensuring staff time, equipment, and technical training as needed

QAA Committee • The QAA committee must meet at least quarterly and as needed to coordinate and evaluate activities under the QAPI Program • Regularly review and analyze data collected to make improvements. • A facility must maintain a quality assessment and assurance (QAA) committee consisting at a minimum of o Director of nursing services o The Medical Director or his/her designee o At least three other members of the facility staff, at least one of whom must be the administrator, owner, a board member, or other individual in a leadership role o The Infection Control and Prevention Officer (11/2019)

What is the QAPI Plan? • A QAPI plan is the written plan containing the process that will guide the nursing home’s efforts in assuring care and services are maintained at acceptable levels of performance and continually improved. • The plan describes how the facility will conduct its required QAPI and QAA committee functions. • Each nursing home, including facilities which are a part of a multichain organization, should tailor its QAPI plan to reflect the specific units, programs, departments, and unique population it serves, as identified in its facility assessment.

Key Components of the QAPI Plan Tracking and measuring performance Establishing goals and thresholds for performance measurement Identifying and prioritizing quality deficiencies Systematically analyzing underlying causes of systemic quality deficiencies • Developing and implementing corrective action or performance improvement activities • Monitoring or evaluating the effectiveness of corrective action/performance improvement activities, and revising as needed. • •

QAPI Plan I. QAPI Goals: What do we want to accomplish? • Goals should be specific, measurable, actionable and relevant • Need timeline for completion II. Scope: How QAPI will be integrated into all aspects of care and services III. Governance and Leadership: QAPI Structure • How responsibilities of top-level leadership are integrated into QAPI • Ensure that QAPI activities receive adequate resources IV. Feedback, Data Systems, Monitoring • How data will be collected analyzed

QAPI Plan V. Guidelines for PIPs • Criteria for prioritizing and selecting PIPs • Team formation • Report format • Documentation of PIP activity and findings VI. Systematic Analysis and Systemic Action • Focus on ways to bring about improvement VII. Communications • Methods of communicating what was learned • Who should receive the information? VIII. Evaluation

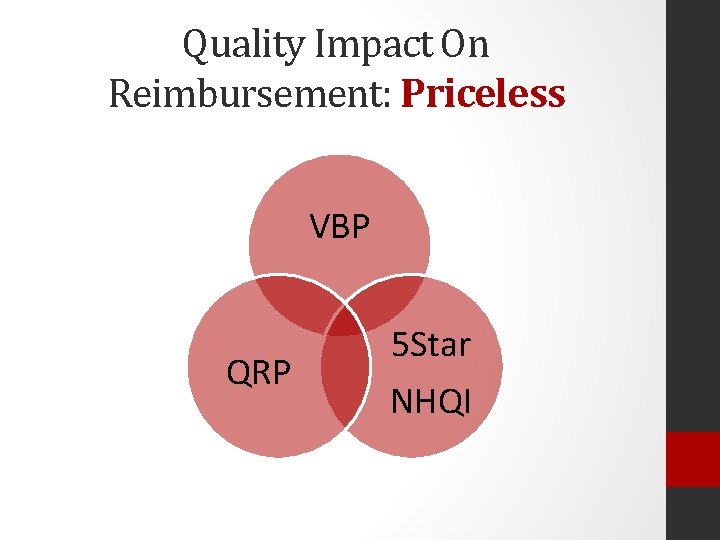
Quality Impact On Reimbursement: Priceless VBP QRP 5 Star NHQI

Performance Improvement • The proactive, continuous study of processes with the intent of preventing or decreasing the likelihood of reoccurrence of issues through identification of opportunities for fixing underlying causes of persistent problems • Performance improvement activities must track medical errors and adverse events, analyze their causes, implement preventive actions and mechanisms that include feedback and learning

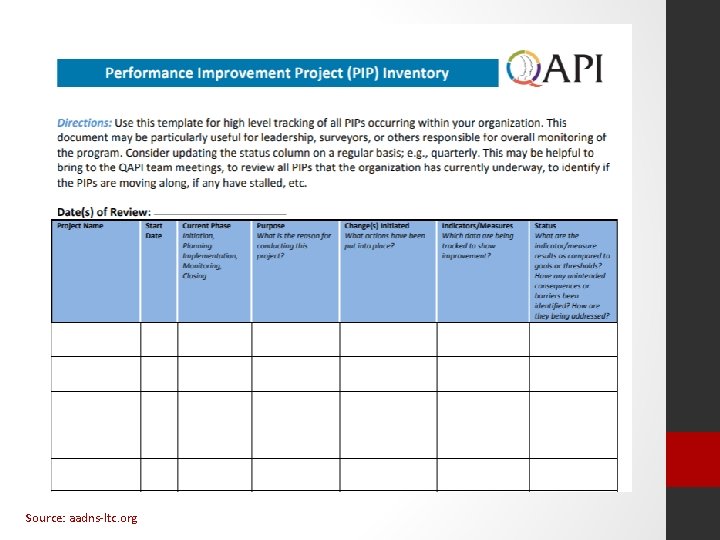
What is a PIP? Performance Improvement Projects are concentrated effort on a particular problem identified in one area of the facility or facilitywide • All identified problems need attention but not all require a PIP • Select PIPs based on high-risk, high-volume and/or problem prone areas in your facility

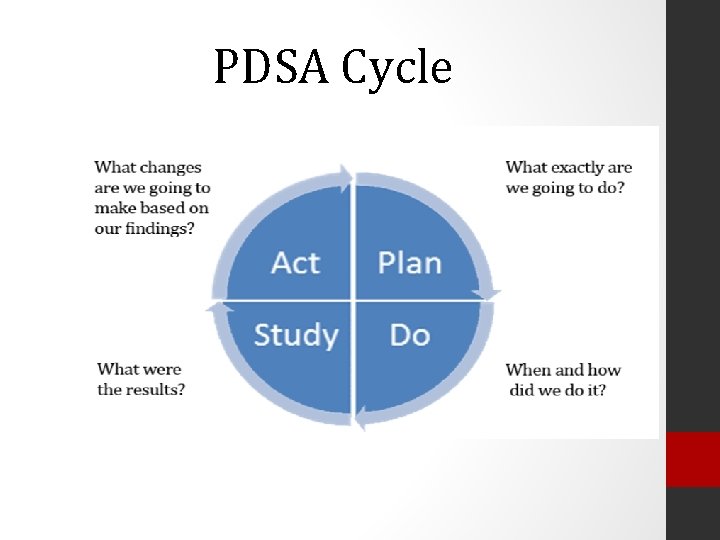
PDSA Cycle

Root Cause Analysis & Contributing Factors • The team needs to clearly understand what went wrong in order to systematically find out why it went wrong. Human factors: communication, training, distraction or bias Rules, policies, or procedures: Was there a problem with current policies procedures? Are there no policies/procedures for addressing this particular issue? Environment/Equipment Barriers: Was this a breakdown in a barrier or a defensive mechanism that was intended to prevent the problem/

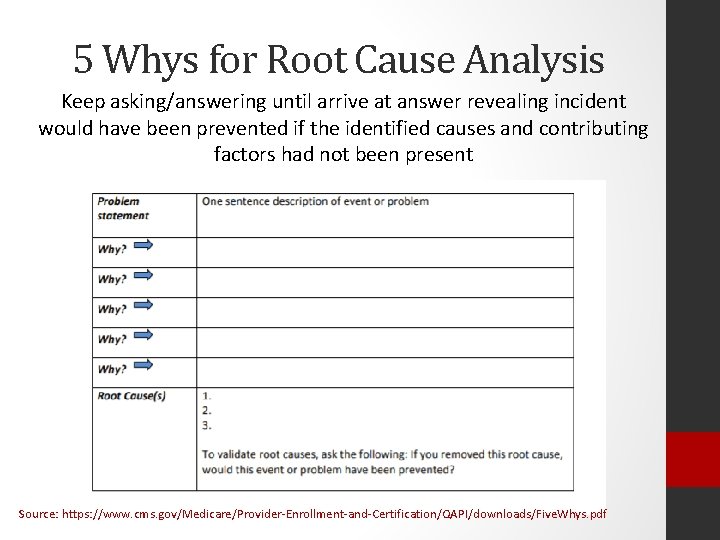
5 Whys for Root Cause Analysis Keep asking/answering until arrive at answer revealing incident would have been prevented if the identified causes and contributing factors had not been present Source: https: //www. cms. gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/Five. Whys. pdf

• Problem: In June, Nursing Home X began to see an increase in pressure injuries among its high-risk residents (> 10%) • Root Cause Analysis: • Why? Residents at risk for developing pressure injuries were not being identified on admission. • Why? Complete body assessments were not always completed on admission. In other instances, residents at risk were not properly captured on the admission assessment. • Why? Staff members completing the admissions were not very familiar with the facility’s admission process and protocol. • Why? During the summer months, the facility was experiencing a surge of call-outs and multiple FT staff on vacation at the same time.

• Goals: 1) By August, 100% of new admissions will have a comprehensive pressure injury risk assessment completed. 2) New admissions who are determined to be high risk will be followed by the wound care nurse on a weekly basis and reviewed at the morning QA meeting. 3) By September, 100% of high risk residents will have the appropriate preventive devices in place. 4) The occurrence of pressure injuries in high-risk residents will be less than 5% by December. • Interventions: 1) Redesign admissions packet to include the comprehensive pressure injury risk assessment form, to be completed within a resident’s first 24 hours of admission 2) Require a half day in-service training for all nursing assistants and licensed nursing staff on assessment for pressure injury risk and prevention, including education on preventive devices. 3) Review all new admissions at the morning QA meeting.

Source: aadns-ltc. org

Helpful Links QAPI Five Elements • https: //www. cms. gov/Medicare/Provider-Enrollment-and. Certification/QAPI/downloads/qapifiveelements. pdf Performance Improvement Project Charter • https: //www. cms. gov/Medicare/Provider-Enrollment-and. Certification/QAPI/downloads/PIPCharter. Wkshtdebedits. pdf Prioritization Worksheet for PIPs • https: //www. cms. gov/Medicare/Provider-Enrollment-and. Certification/QAPI/downloads/PIPPrior. Wkshtdebedits. pdf QAPI Goal Setting Worksheet • https: //www. cms. gov/Medicare/Provider-Enrollment-and. Certification/QAPI/downloads/QAPIGoal. Setting. pdf PIP Inventory • https: //www. cms. gov/Medicare/Provider-Enrollment-and. Certification/QAPI/downloads/PIPInventorydebedits. pdf 5 Whys • https: //www. cms. gov/Medicare/Provider-Enrollment-and. Certification/QAPI/downloads/Five. Whys. pdf