Protecting Human Participants in Research Contact Information Susanne


































- Slides: 35

Protecting Human Participants in Research

Contact Information Susanne Santi Senior Manager, Research Ethics 1027 Needles Hall 519 -888 -4567 extension 37163 ssanti@uwaterloo. ca 2 Research with Humans

Overview § § § § 3 Canadian Research Ethics Guidelines Architecture Research Methods Recruitment Informed Consent Data Collection, Storage and Retention ORE Review and Application Processes Responsibility of Researchers Research with Humans

Canadian Research Ethics Guidelines Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS) 4 Research with Humans

Context of Ethics Framework § § 5 Need for Research: advance knowledge to benefit groups and society Respect for Human Dignity: ethic should include (1) morally acceptable ends, (2) morally acceptable means to those ends Subject-Centred Perspective: participants usually central to a study; not treated as objects Guiding Ethical Principles Research with Humans

TCPS – Guiding Principles § § § § 6 Respect for human dignity Respect for free and informed consent Respect for vulnerable persons Respect for privacy and confidentiality Respect for justice and inclusiveness Balancing harms and benefits Minimizing harm Maximizing benefits Research with Humans

TCPS – Article 1. 1 All research that involves living human subjects requires review and approval by an REB (research ethics board) in accordance with this Policy Statement, before the research is started… 7 Research with Humans

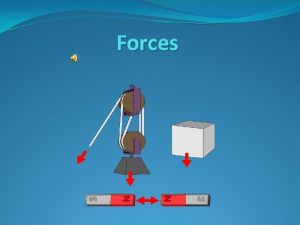
Architecture Research Methods Research involving human participants may involve § Observation § Photography § Interview § Focus group § Survey (questionnaire) 8 Research with Humans

Architecture Research Methods Usually, these procedures § do not involve physical contact between researcher and participant § do not involve interference with behaviours § do not involve deception Risks due to procedures generally negligible, but may increase due to context, lack of confidentiality 9 Research with Humans

Participant Observation § § Public place – no informed consent Not public place – informed consent Observing behaviour does not involve recording conversations or video recording. 10 Research with Humans

Photographs § § § 11 Publicly accessible space People are not identifiable – no consent to use People are identifiable – obtain consent to use photo in research publications Research with Humans

12 Research with Humans

Interview, Focus Group, Survey Fully Inform participants about the study, and obtain written consent Unless it is an anonymous survey 13 Research with Humans

Interviews Key Informant § Professionals in design, planning, etc. § May be difficult to provide anonymity – key agents can be traced § Recognize expert opinion, contribution by identifying in publications, with permission; provide opportunity to review quotations § Obtain permission to audio record 14 Research with Humans

Interview Users or Citizen Participants in Design 15 § Anonymity should be provided § Participant characteristics – age, gender, ethnicity, etc. – should not lead to identification § Obtain permission to audio record Research with Humans

Focus Group § § § 16 Anonymity of information reported Researcher guarantees confidentiality; however cannot guarantee confidentiality by session participants Audio or video recording is for analysis purposes; if wish to use for presentation or publication, obtain written consent from all session participants Research with Humans

Survey (Questionnaire) § § § 17 Paper and pencil, web-based, in-person, telephone Usually, questions with multiple-choice responses, statements with likert scales, ranking of items, and/or short, open-ended response questions Fully inform; no written consent for anonymous survey Research with Humans

Recruitment in Organizations Obtain permission from organization’s gatekeeper to conduct study in organization or recruit participants • discuss project with gatekeeper • work out a recruitment method while protecting privacy of potential participants • script/letter for gatekeeper permission 18 Research with Humans

Recruitment l l Who is recruited – justice and inclusiveness, credibility of findings Participation is voluntary; no coercion or exercise of power or authority For minors, recruitment begins with parents Variety of routes: telephone, email, paper, poster, flyer; see samples on website http: //iris. uwaterloo. ca/ethics/human/application/101 samples. htm 19 Research with Humans

Informed Consent Process Informed consent process involves: l Participant-centered approach l Full disclosure in lay language i. e. told exactly what is going to happen l Interactive- between researcher & participant l Materials should be grammatically correct & easy to read: grade 8 level, sub-titles, Q&A format, white space 20 Research with Humans

Informed Consent: Information-Consent Letter Elements of an Information Consent Letter: ● Names, affiliation, and contact #s for Faculty Supervisor and Student Investigator ● Study purpose ● Lay language description of procedures: examples of the type of questions for interview or questionnaire ● Indicate participants may decline answering any question(s), if interview or questionnaire ● Describe all known or anticipated risks and benefits ● Details of time commitment required for participation 21 Research with Humans

Information-Consent Letter Elements of an Information Consent Letter, Cont’d: ● Free not to participate, or subsequently withdraw consent, without jeopardizing any entitlements ● Details about follow-up sessions or subsequent related project ● Procedures to ensure confidentiality of data and anonymity of participants -- limitations on confidentiality should be noted ● Financial or other remuneration for participation ● Length of retention and security of data 22 Research with Humans

Information-Consent Letter Elements of an Information Consent Letter, Cont’d: ● Opportunity to ask any related questions and receive answers to their satisfaction ● Statement: This study has been reviewed and received ethics clearance through the Office of Research Ethics. However, the final decision about participation is yours. If you have any comments or concerns please contact the office at (519) 888 -4567 ext. 36005 or by email at ssykes@uwaterloo. ca 23 Research with Humans

Data Collection, Storage & Retention l l l 24 Privacy and confidentiality while collecting data Data are kept secure from theft, interception, copying or perusal Personal identifiers removed from questionnaires, tapes, other documents No names/identifiers released without written consent Access to data with identifiers only by researchers Research with Humans

Risks vs. Benefits of Procedures l l 25 Potential benefits of research must outweigh any potential risks Researcher must determine both known and potential risks of procedures Risks of procedures can be physical, psychological, legal, economic and social Risks of procedures assessed within context Research with Humans

UW Ethics Review Process What Research Requires Ethics Review? All research* involving living human participants *Research involves a systematic investigation to establish facts, principles or generalizable knowledge 26 Research with Humans

UW Ethics Review Process Two Ethics Review Routes: l ORE: Ethics review by Director or a Manager, Office of Research Ethics l HREC: Ethics review by all members of Human Research Ethics Committee (or sub -committee) 27 Research with Humans

UW Ethics Review Process How is Ethics Review Route Determined? Most commonly, on the basis of identified level of risks to participants • Applications that pose no more than minimal risk to participants are reviewed by Director or a Manager • Applications that pose greater than minimal risk to participants are referred to the HREC 28 Research with Humans

UW Ethics Review Process What is Minimal Risk? …. Participation in research activities in which the potential risk of harm is no greater than that which participants already experience in their everyday lives. 29 Research with Humans

UW Ethics Review Process Primary Considerations: l Study Details - Purpose, methodology, recruitment, participants l l 30 Recruitment procedures Anonymity of participants and confidentiality of data Risks vs. benefits of procedures Informed consent process Research with Humans

UW, Human Research Accountability l l l 31 Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans University of Waterloo Guidelines for Research with Human Participants Memorandum of Understanding between Federal Granting Agencies and Institutions Research with Humans

ORE Application Process Form 101/101 A 32 Research with Humans

Review Time ● 15 working days for review, may be less ● Obtain Ethics Review Feedback via email – cannot begin project until ethics clearance ● Respond to comments and make revisions ● Responses and revisions returned to OHRAC@uwaterloo. ca ● Review Feedback emails will be sent until all comments addressed, then ethics clearance ● Once ethics clearance can begin project 33 Research with Humans

Researcher’s Responsibilities l l l 34 Researchers expected to design and implement research consistent with TCPS and with UW’s Guidelines Researchers ensure all their research involving humans undergoes ethics review and receives ethics clearance prior to commencement of the project Researchers conduct research in accordance with their description in the application for which ethics clearance has been granted Research with Humans

Researcher’s Responsibilities l l l 35 Researchers submit all subsequent modifications to the protocol for ethics review and clearance before changes are undertaken (ORE 104) Researchers responsible for submitting an annual Progress Report for all ongoing research projects (ORE 105) Researchers submit an adverse event form for any events related to the procedures used that adversely affect participants (ORE 106) Research with Humans
 Example of participants section in research paper
Example of participants section in research paper Citi training cmu
Citi training cmu Which force
Which force Which of the following is sliding contact bearing
Which of the following is sliding contact bearing Non contact force definition
Non contact force definition Non-contact forces portfolio
Non-contact forces portfolio Dermatitis atopica icd 10
Dermatitis atopica icd 10 Contact vs noncontact forces
Contact vs noncontact forces Thermoelectric cooler
Thermoelectric cooler Post encounter stage
Post encounter stage Contact vs noncontact forces
Contact vs noncontact forces Air resistance contact force
Air resistance contact force Obtaining and protecting your credit
Obtaining and protecting your credit Chapter 9 obtaining and protecting your credit
Chapter 9 obtaining and protecting your credit Chapter 20 civil liberties protecting individual rights
Chapter 20 civil liberties protecting individual rights Reactions of aldehydes and ketones summary
Reactions of aldehydes and ketones summary Primary aldehyde
Primary aldehyde Carbamate protecting group
Carbamate protecting group Bitter woman quotes
Bitter woman quotes Chapter 9 obtaining and protecting your credit
Chapter 9 obtaining and protecting your credit Protecting student data
Protecting student data Protecting consumers savers and investors examples
Protecting consumers savers and investors examples Which of the following is not part of overall biodiversity
Which of the following is not part of overall biodiversity Single species approaches to protecting biodiversity
Single species approaches to protecting biodiversity Chapter 20 civil liberties protecting individual rights
Chapter 20 civil liberties protecting individual rights Chapter 20 civil liberties protecting individual rights
Chapter 20 civil liberties protecting individual rights Human contact
Human contact Contact information title
Contact information title Title contact information
Title contact information Janie bean
Janie bean Susanne wischmeyer
Susanne wischmeyer Perineumplastiek
Perineumplastiek Susanne shultz
Susanne shultz Susanne augenhofer
Susanne augenhofer Susanne dufva
Susanne dufva Fördelar med kejsarsnitt
Fördelar med kejsarsnitt