Prokaryotic Expression SDSPAGE Western Dr Sameera Hassan Assistant









- Slides: 23

Prokaryotic Expression, SDS-PAGE & Western Dr. Sameera Hassan Assistant Professor


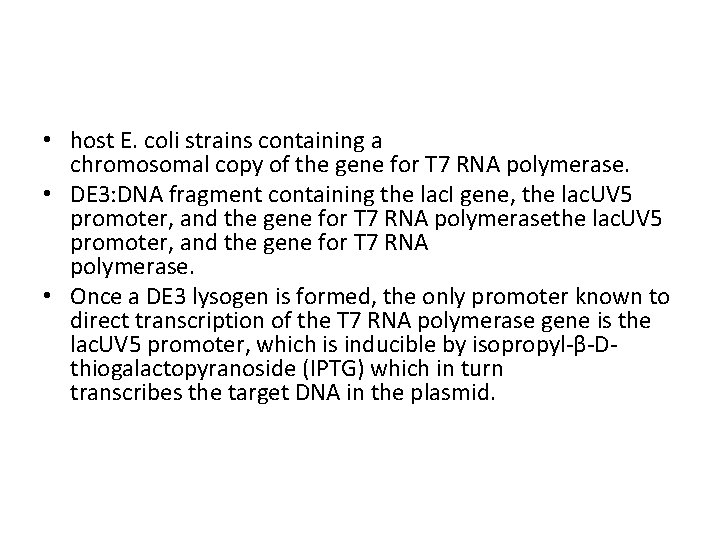
• host E. coli strains containing a chromosomal copy of the gene for T 7 RNA polymerase. • DE 3: DNA fragment containing the lac. I gene, the lac. UV 5 promoter, and the gene for T 7 RNA polymerase. • Once a DE 3 lysogen is formed, the only promoter known to direct transcription of the T 7 RNA polymerase gene is the lac. UV 5 promoter, which is inducible by isopropyl-β-Dthiogalactopyranoside (IPTG) which in turn transcribes the target DNA in the plasmid.


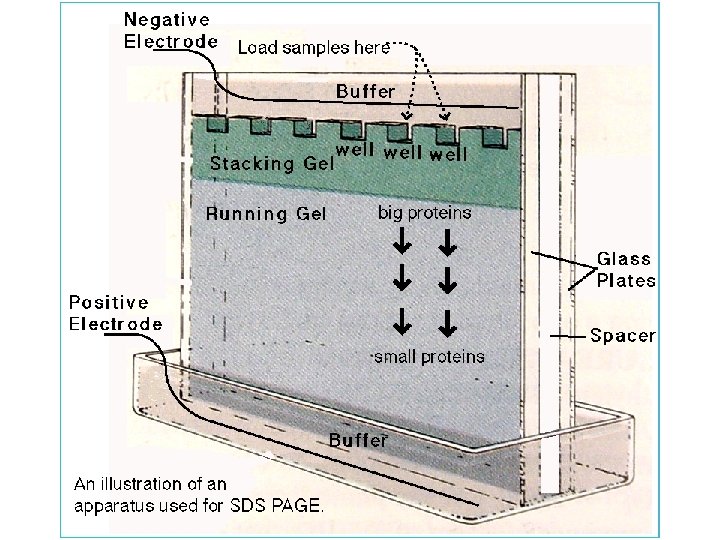
Protein Electrophoresis • Separate proteins based on – Size (Molecular Weight - MW) • Allows us to – characterize – quantify – determine purity of sample – compare proteins from different sources • And it is a step in Western blot

Procedure • • • Prepare polyacrylamide gels Add diluted samples to the sample buffer Heat to 95 C for 4 minutes Load the samples onto polyacrylamide gel Run at 200 volts for 30 -40 minutes Stain

Acrylamide • Acrylamide is a neurotoxin. • The acrylamide concentration of the gel can a be varied, generally in the range from 5% to 25%.


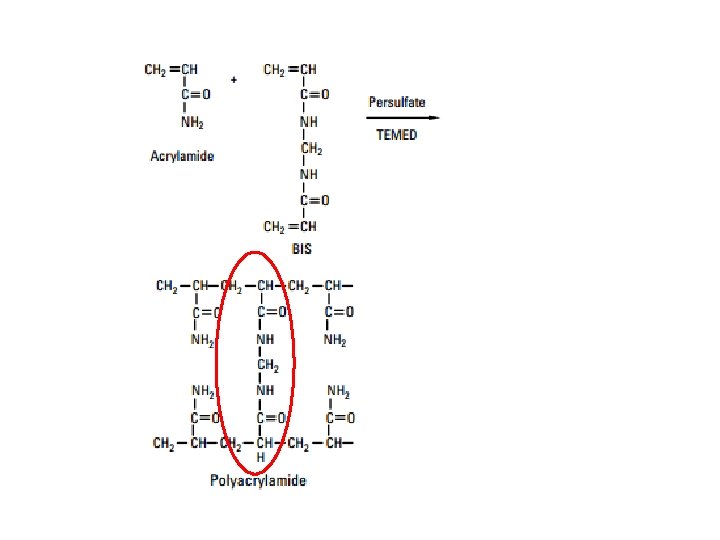
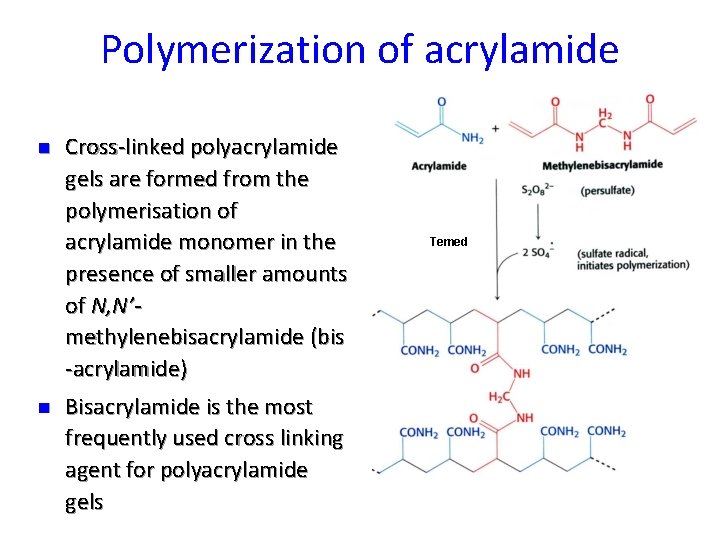
Polymerization of acrylamide n n Cross-linked polyacrylamide gels are formed from the polymerisation of acrylamide monomer in the presence of smaller amounts of N, N’methylenebisacrylamide (bis -acrylamide) Bisacrylamide is the most frequently used cross linking agent for polyacrylamide gels Temed

Vertical Gel Format: Polyacrylamide Gel Electrophoresis Reservoir/Tank Power Supply Glass Plates, Spacers, and Combs

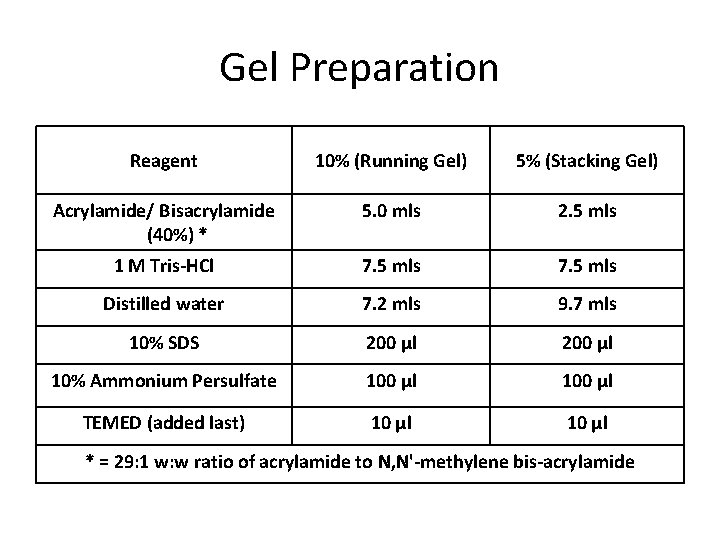
Gel Preparation Reagent 10% (Running Gel) 5% (Stacking Gel) Acrylamide/ Bisacrylamide (40%) * 5. 0 mls 2. 5 mls 1 M Tris-HCl 7. 5 mls Distilled water 7. 2 mls 9. 7 mls 10% SDS 200 µl 10% Ammonium Persulfate 100 µl TEMED (added last) 10 µl * = 29: 1 w: w ratio of acrylamide to N, N'-methylene bis-acrylamide


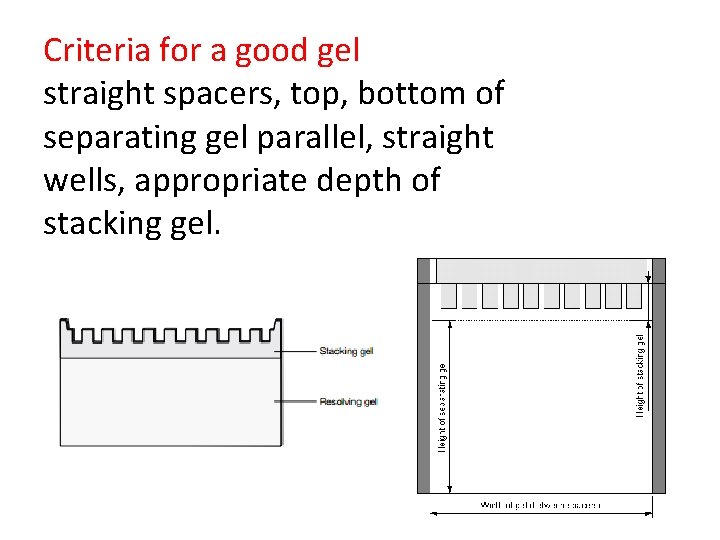
Criteria for a good gel straight spacers, top, bottom of separating gel parallel, straight wells, appropriate depth of stacking gel.

• A typical mini-gel well holds 10 µl easily, and perhaps 20 µl • To completely denature the samples we heat them in a steaming water bath for at least 10 minutes.

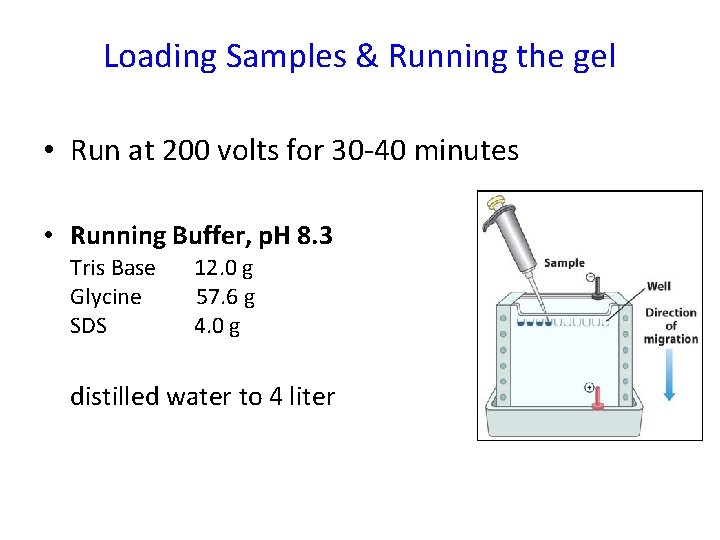
Loading Samples & Running the gel • Run at 200 volts for 30 -40 minutes • Running Buffer, p. H 8. 3 Tris Base 12. 0 g Glycine 57. 6 g SDS 4. 0 g distilled water to 4 liter

• When the dye front is nearly at the bottom of the gel it is time to stop the run.

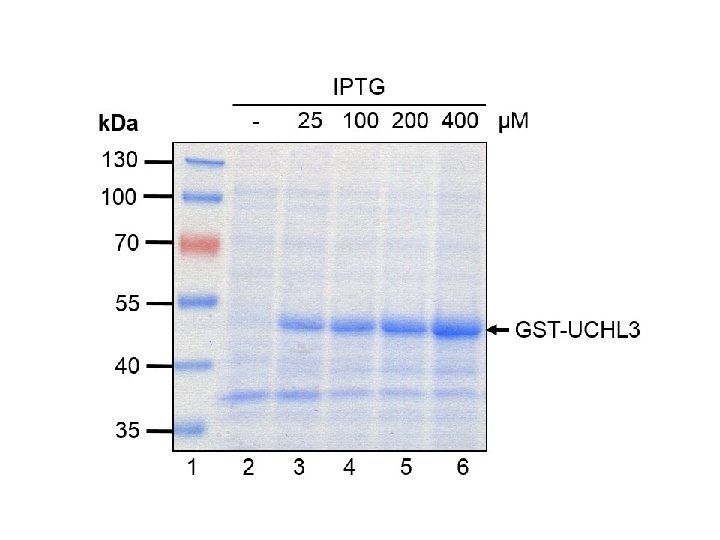
• commonly used stain for detecting proteins in polyacrylamide gels is 0. 1% Coomassie Blue dye in 50% methanol, 10% glacial acetic acid. • Detection limit is 50 ng protein band • The dye actually penetrates the entire gel, however it only sticks permanently to the proteins. • Excess dye is washed out by 'destaining' with acetic acid/methanol, also with agitation.

• Properly stained/destained gels display a pattern of blue protein bands against a clear background. • The gels can be dried down or photographed for later analysis and documentation.

Blocking • Blocking is a very important step in the immunodetection phase of Western blotting because it prevents non-specific binding of antibody to the blotting membrane • The most commonly used blocking solutions contain 3 -5% BSA or non-fat dried milk in a solution of PBS (phosphate buffered saline) or TBS (tris buffered saline) • Often, a small amount of Tween 20 detergent is added to blocking and washing solutions to reduce background staining, and the buffer is known as PBST or TBST

Antibody Probing • Once the protein samples are separated and transferred onto a membrane, the protein of interest is detected and localized using a specific antibody • The blot will be incubated in a dilute solution of antibody, usually for a few hours at room temperature or overnight at 4°C • The antibody is diluted in wash buffer (PBST or TBST) or in the blocking solution, the choice depends upon the antibody • Since antibody preparations vary in their levels of purity and specific binding properties, there will be differences in the level of dilution required • The manufacturer’s datasheet should provide dilution recommendations for a particular preparation

Antibody Probing • Usually, Western blotting protocols utilize a non-labeled primary antibody directed against the target protein • Wash the membrane several times in TBST while agitating, 5 minutes or more per wash, to remove residual primary antibody • A species-specific, labeled secondary antibody directed against the constant region of the primary antibody is then used • The secondary antibody serves not only as a carrier of the label but is also a mechanism to amplify the emitted signals, as many secondary antibodies can theoretically bind simultaneously to the primary antibody • Secondary Ab is also diluted according to the manufacturer’s recommendations and incubated for 1 hour at RT

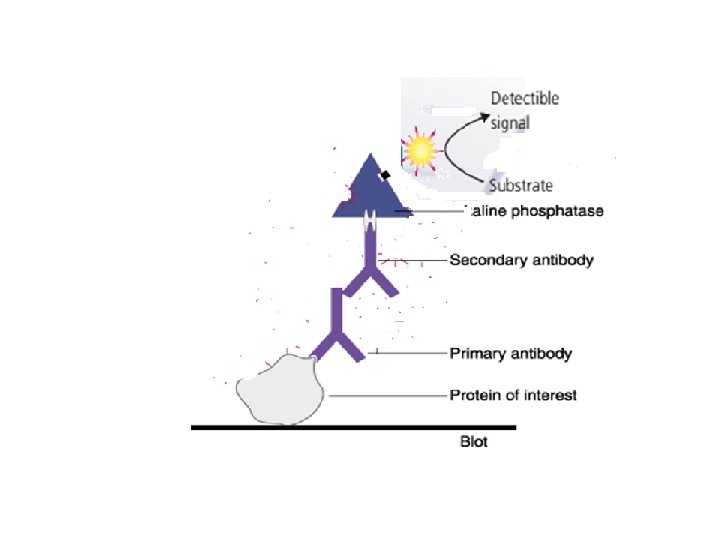
6 - Detection with Substrate • The most common antibody label used in Western blots is AP, a small, stable enzyme with high specificity and rapid turnover • The signal is detected when HRP is exposed to a substrate solution in the final step of the immunodetection procedure • Substrate solutions for Western blotting are chemical reagents that are acted upon by the enzyme to yield a signal that can be easily measured
