PPAR activation Impact on pathways of clinical care





- Slides: 21

PPAR activation Impact on pathways of clinical care

New paradigm of multiple risk factormanagement The future of drug therapy belongs to prevention, which is just now being addressed, and to intensive management of all cardiovascular risk factors Kaplan NM. Hypertension. 2005; 46: 257 -8.

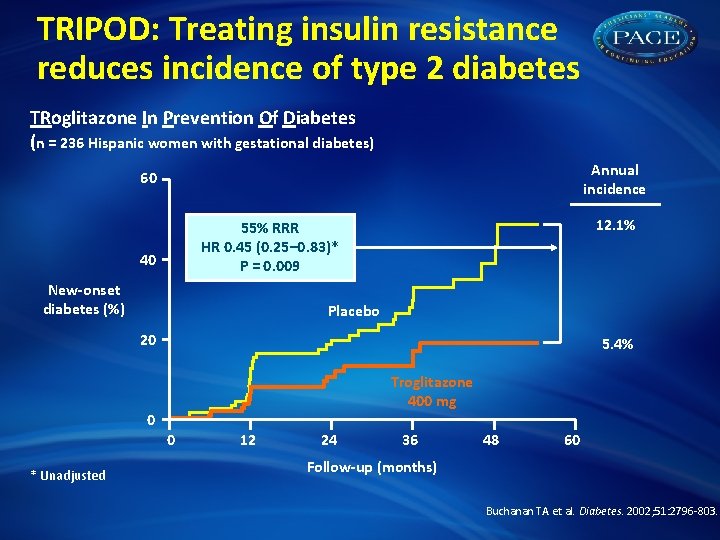
TRIPOD: Treating insulin resistance reduces incidence of type 2 diabetes TRoglitazone In Prevention Of Diabetes (n = 236 Hispanic women with gestational diabetes) Annual incidence 60 12. 1% 55% RRR HR 0. 45 (0. 25– 0. 83)* P = 0. 009 40 New-onset diabetes (%) Placebo 20 5. 4% Troglitazone 400 mg 0 0 * Unadjusted 12 24 36 48 60 Follow-up (months) Buchanan TA et al. Diabetes. 2002; 51: 2796 -803.

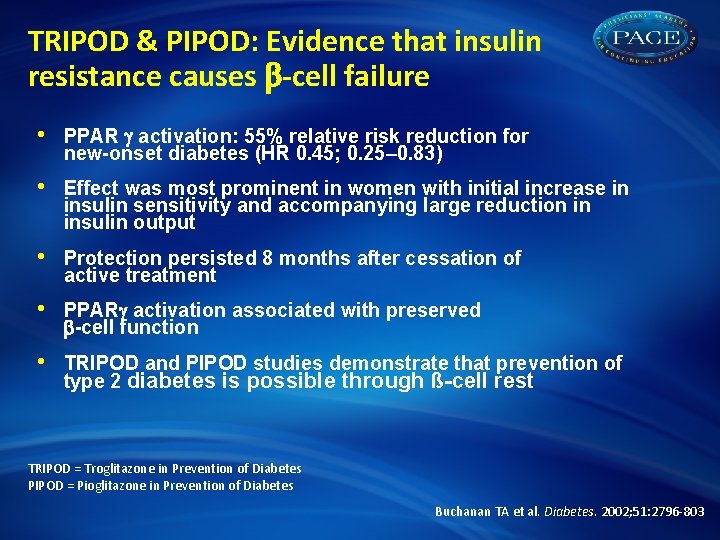
TRIPOD & PIPOD: Evidence that insulin resistance causes -cell failure • PPAR activation: 55% relative risk reduction for new-onset diabetes (HR 0. 45; 0. 25– 0. 83) • Effect was most prominent in women with initial increase in insulin sensitivity and accompanying large reduction in insulin output • Protection persisted 8 months after cessation of active treatment • PPAR activation associated with preserved -cell function • TRIPOD and PIPOD studies demonstrate that prevention of type 2 diabetes is possible through ß-cell rest TRIPOD = Troglitazone in Prevention of Diabetes PIPOD = Pioglitazone in Prevention of Diabetes Buchanan TA et al. Diabetes. 2002; 51: 2796 -803

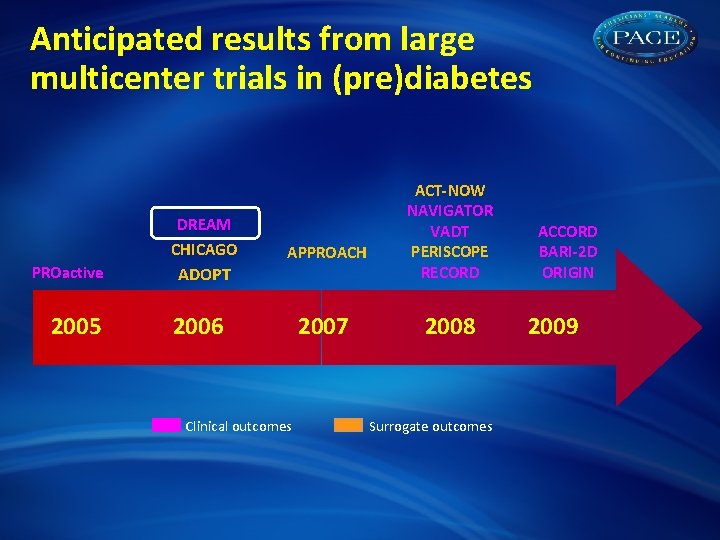
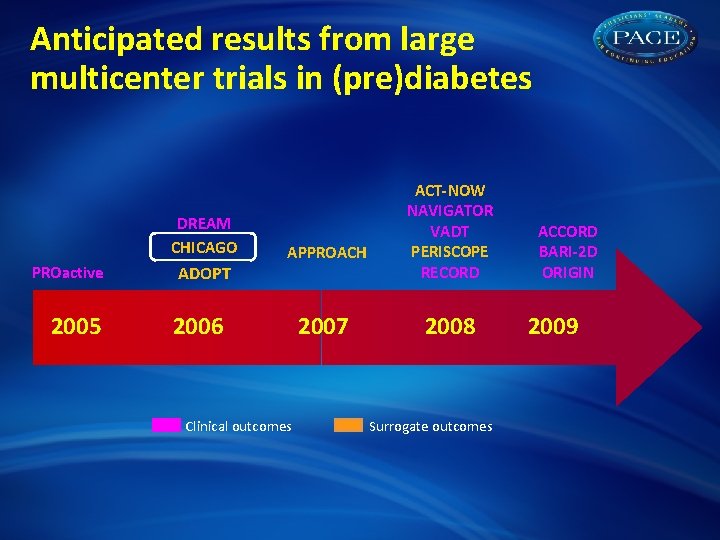
Anticipated results from large multicenter trials in (pre)diabetes DREAM CHICAGO PROactive 2005 APPROACH ADOPT 2006 Clinical outcomes 2007 ACT-NOW NAVIGATOR VADT PERISCOPE RECORD 2008 Surrogate outcomes ACCORD BARI-2 D ORIGIN 2009

DREAM Background and study objective Diabetes REduction Assessment with ramipril and rosiglitazone Medication • Previous studies have shown evidence for new-onset diabetes with RAAS and PPAR agonists • Does treatment with ramipril and/or rosiglitazone prevent or delay the development of diabetes in persons with IGT or IFG and no diabetes? DREAM Trial Investigators. Diabetologia. 2004; 47: 1519 -27.

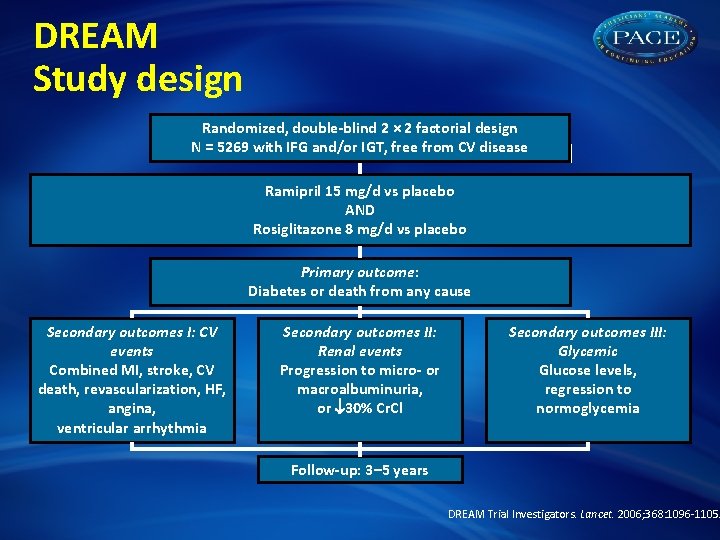
DREAM Study design Randomized, double-blind 2 × 2 factorial design N = 5269 with IFG and/or IGT, free from CV disease Ramipril 15 mg/d vs placebo AND Rosiglitazone 8 mg/d vs placebo Primary outcome: Diabetes or death from any cause Secondary outcomes I: CV events Combined MI, stroke, CV death, revascularization, HF, angina, ventricular arrhythmia Secondary outcomes II: Renal events Progression to micro- or macroalbuminuria, or 30% Cr. Cl Secondary outcomes III: Glycemic Glucose levels, regression to normoglycemia Follow-up: 3– 5 years DREAM Trial Investigators. Lancet. 2006; 368: 1096 -1105.

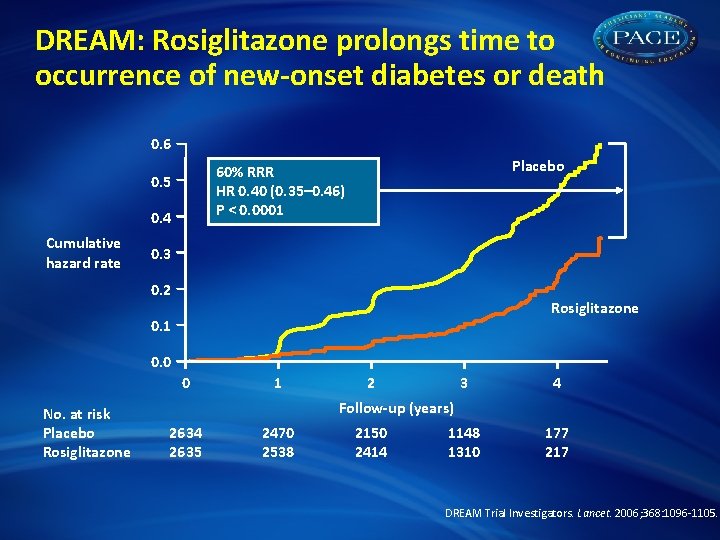
DREAM: Rosiglitazone prolongs time to occurrence of new-onset diabetes or death 0. 6 0. 5 0. 4 Cumulative hazard rate Placebo 60% RRR HR 0. 40 (0. 35– 0. 46) P < 0. 0001 0. 3 0. 2 Rosiglitazone 0. 1 0. 0 0 No. at risk Placebo Rosiglitazone 1 2 3 4 1148 1310 177 217 Follow-up (years) 2634 2635 2470 2538 2150 2414 DREAM Trial Investigators. Lancet. 2006; 368: 1096 -1105.

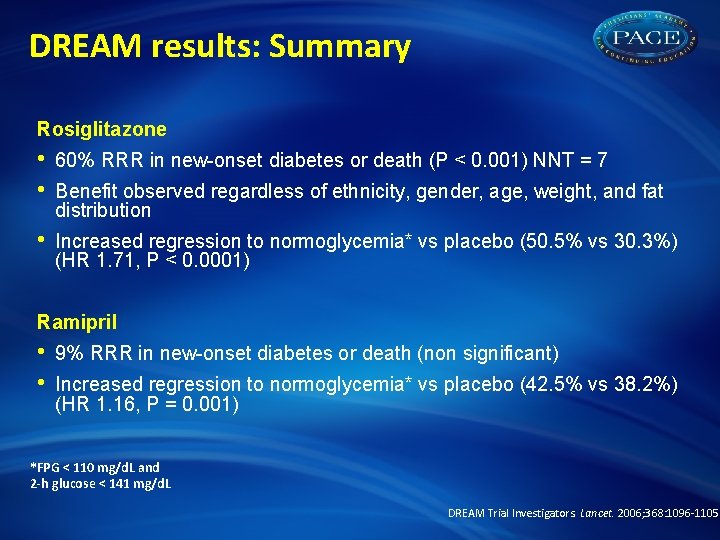
DREAM results: Summary Rosiglitazone • • 60% RRR in new-onset diabetes or death (P < 0. 001) NNT = 7 • Increased regression to normoglycemia* vs placebo (50. 5% vs 30. 3%) (HR 1. 71, P < 0. 0001) Benefit observed regardless of ethnicity, gender, age, weight, and fat distribution Ramipril • • 9% RRR in new-onset diabetes or death (non significant) Increased regression to normoglycemia* vs placebo (42. 5% vs 38. 2%) (HR 1. 16, P = 0. 001) *FPG < 110 mg/d. L and 2 -h glucose < 141 mg/d. L DREAM Trial Investigators. Lancet. 2006; 368: 1096 -1105

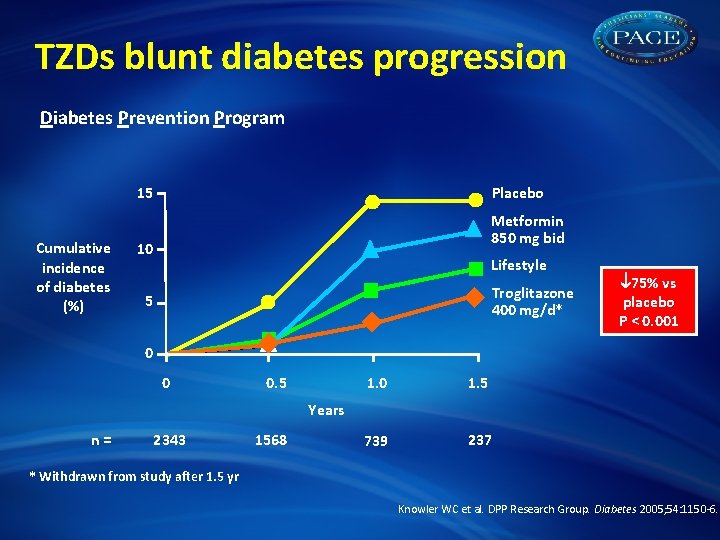
TZDs blunt diabetes progression Diabetes Prevention Program 15 Cumulative incidence of diabetes (%) Placebo Metformin 850 mg bid 10 Lifestyle Troglitazone 400 mg/d* 5 75% vs placebo P < 0. 001 0 0 0. 5 1. 0 1. 5 739 237 Years n= 2343 1568 * Withdrawn from study after 1. 5 yr Knowler WC et al. DPP Research Group. Diabetes 2005; 54: 1150 -6.

Anticipated results from large multicenter trials in (pre)diabetes DREAM CHICAGO PROactive 2005 ADOPT APPROACH 2006 Clinical outcomes 2007 ACT-NOW NAVIGATOR VADT PERISCOPE RECORD 2008 Surrogate outcomes ACCORD BARI-2 D ORIGIN 2009

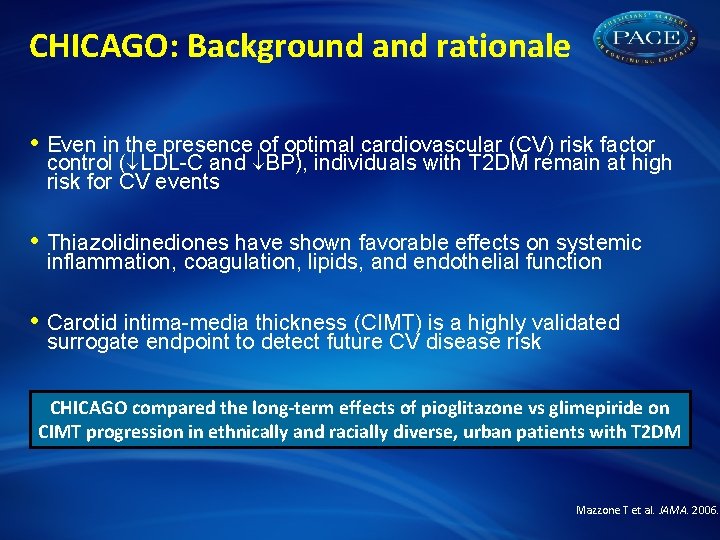
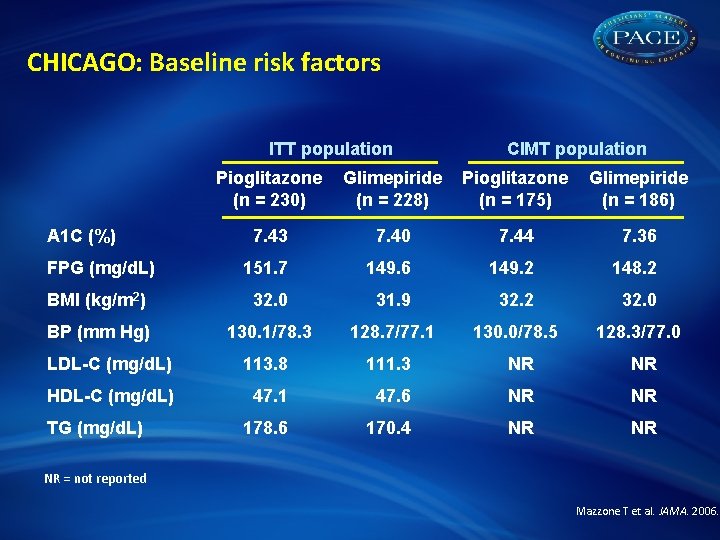
CHICAGO: Background and rationale • Even in the presence of optimal cardiovascular (CV) risk factor control ( LDL-C and BP), individuals with T 2 DM remain at high risk for CV events • Thiazolidinediones have shown favorable effects on systemic inflammation, coagulation, lipids, and endothelial function • Carotid intima-media thickness (CIMT) is a highly validated surrogate endpoint to detect future CV disease risk CHICAGO compared the long-term effects of pioglitazone vs glimepiride on CIMT progression in ethnically and racially diverse, urban patients with T 2 DM Mazzone T et al. JAMA. 2006.

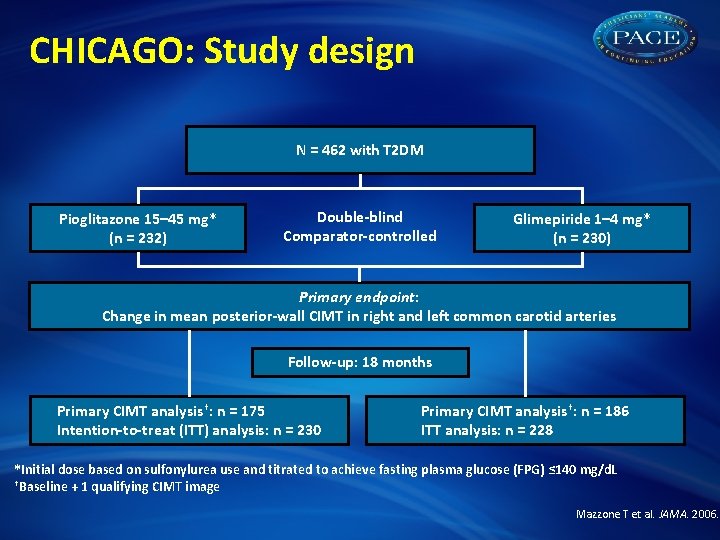
CHICAGO: Study design N = 462 with T 2 DM Pioglitazone 15– 45 mg* (n = 232) Double-blind Comparator-controlled Glimepiride 1– 4 mg* (n = 230) Primary endpoint: Change in mean posterior-wall CIMT in right and left common carotid arteries Follow-up: 18 months Primary CIMT analysis†: n = 175 Intention-to-treat (ITT) analysis: n = 230 Primary CIMT analysis†: n = 186 ITT analysis: n = 228 *Initial dose based on sulfonylurea use and titrated to achieve fasting plasma glucose (FPG) ≤ 140 mg/d. L †Baseline + 1 qualifying CIMT image Mazzone T et al. JAMA. 2006.

CHICAGO: Baseline risk factors ITT population CIMT population Pioglitazone (n = 230) Glimepiride (n = 228) Pioglitazone (n = 175) Glimepiride (n = 186) 7. 43 7. 40 7. 44 7. 36 FPG (mg/d. L) 151. 7 149. 6 149. 2 148. 2 BMI (kg/m 2) 32. 0 31. 9 32. 2 32. 0 BP (mm Hg) 130. 1/78. 3 128. 7/77. 1 130. 0/78. 5 128. 3/77. 0 LDL-C (mg/d. L) 113. 8 111. 3 NR NR HDL-C (mg/d. L) 47. 1 47. 6 NR NR 178. 6 170. 4 NR NR A 1 C (%) TG (mg/d. L) NR = not reported Mazzone T et al. JAMA. 2006.

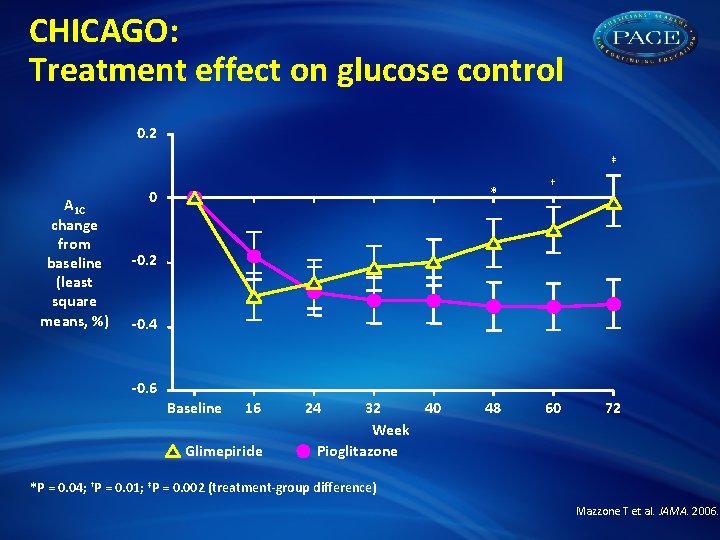
CHICAGO: Treatment effect on glucose control 0. 2 ‡ A 1 C change from baseline (least square means, %) * 0 † -0. 2 -0. 4 -0. 6 Baseline 16 Glimepiride 24 32 40 Week Pioglitazone 48 60 72 *P = 0. 04; †P = 0. 01; ‡P = 0. 002 (treatment-group difference) Mazzone T et al. JAMA. 2006.

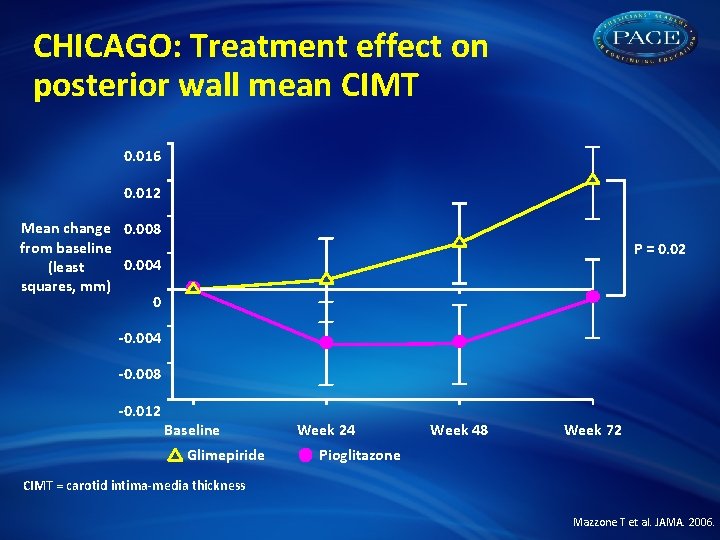
CHICAGO: Treatment effect on posterior wall mean CIMT 0. 016 0. 012 Mean change 0. 008 from baseline 0. 004 (least squares, mm) 0 P = 0. 02 -0. 004 -0. 008 -0. 012 Baseline Glimepiride Week 24 Week 48 Week 72 Pioglitazone CIMT = carotid intima-media thickness Mazzone T et al. JAMA. 2006.

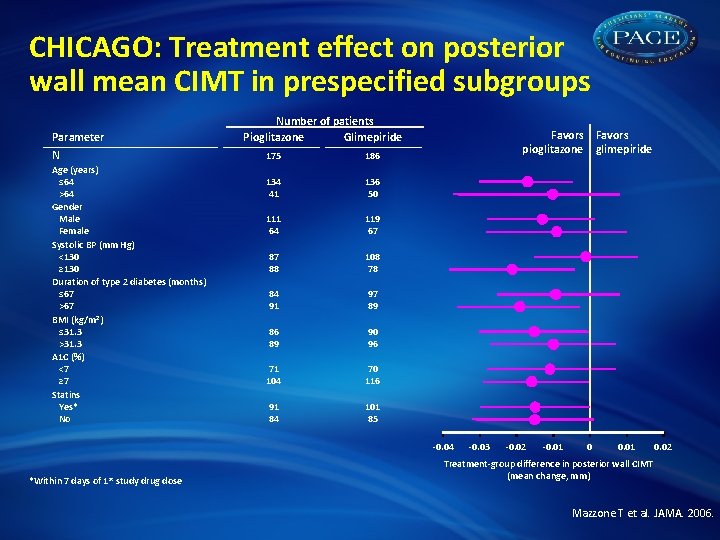
CHICAGO: Treatment effect on posterior wall mean CIMT in prespecified subgroups Parameter N Age (years) ≤ 64 >64 Gender Male Female Systolic BP (mm Hg) <130 ≥ 130 Duration of type 2 diabetes (months) ≤ 67 >67 BMI (kg/m 2) ≤ 31. 3 >31. 3 A 1 C (%) <7 ≥ 7 Statins Yes* No Number of patients Pioglitazone Glimepiride 175 186 134 41 136 50 111 64 119 67 87 88 108 78 84 91 97 89 86 89 90 96 71 104 70 116 91 84 101 85 Favors pioglitazone glimepiride -0. 04 *Within 7 days of 1 st study drug dose -0. 03 -0. 02 -0. 01 0. 02 Treatment-group difference in posterior wall CIMT (mean change, mm) Mazzone T et al. JAMA. 2006.

CHICAGO: Summary • In an ethnically and racially diverse patient population with T 2 DM, treatment with pioglitazone demonstrated clinical benefits: –Progression of carotid atherosclerosis was retarded vs sulfonylurea (P = 0. 02) –Benefits observed across all prespecified subgroups: age, gender, SBP, diabetes duration, BMI, Hb. A 1 C, statin use • Edema and weight gain were higher in TZD group • CIMT may be preferred for assessing treatment-related changes in carotid atherosclerosis Mazzone T et al. JAMA. 2006. Bernard S et al. Diabetes Care. 2005; 28: 1158 -62.

CHICAGO: Implications • Compared with previous trial cohorts, patients in CHICAGO were welltreated at baseline and had near-optimal risk factor control: – Mean LDL-C 113. 8 mg/d. L (pioglitazone) and 111. 3 mg/d. L (glimepiride) – 130. 1/78. 3 mm Hg (pioglitazone) and 128. 7/77. 1 mm Hg (glimepiride) • Slowed atherosclerosis progression is consistent with clinical endpoint data reported in PROactive • Results are similar to those by Langenfeld et al. , who also showed pioglitazone was associated with a greater reduction in carotid IMT at 24 weeks compared with glimepiride in diabetic patients • Additional data will contribute to the overall understanding and clinical significance of CHICAGO results Mazzone T et al. JAMA. 2006. Dormandy JA et al. Lancet. 2005; 366: 1279 -89. Langenfeld et al. Circulation 2005; 111: 2525 -31.

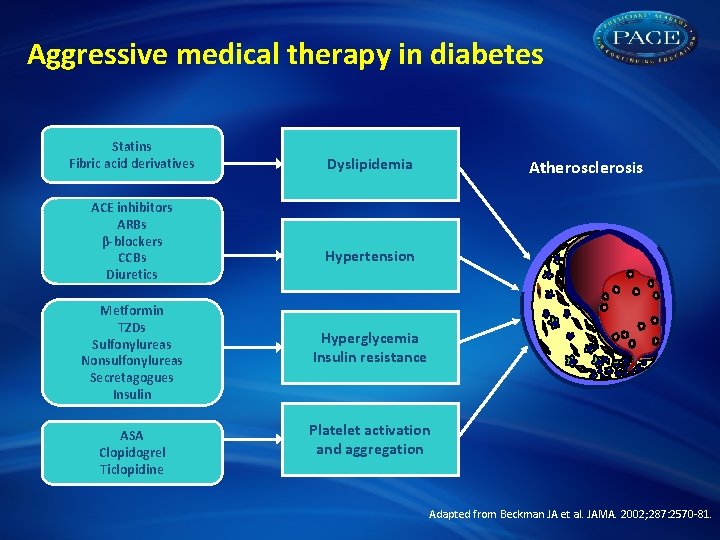
Aggressive medical therapy in diabetes Statins Fibric acid derivatives ACE inhibitors ARBs β-blockers CCBs Diuretics Metformin TZDs Sulfonylureas Nonsulfonylureas Secretagogues Insulin ASA Clopidogrel Ticlopidine Dyslipidemia Atherosclerosis Hypertension Hyperglycemia Insulin resistance Platelet activation and aggregation Adapted from Beckman JA et al. JAMA. 2002; 287: 2570 -81.

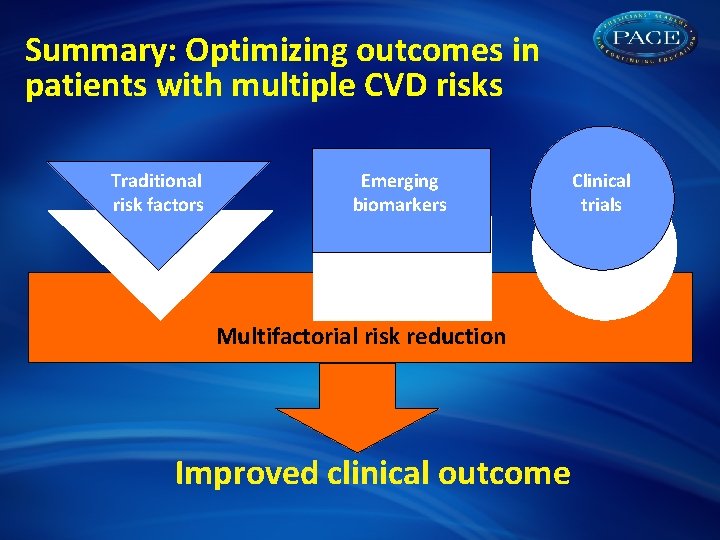
Summary: Optimizing outcomes in patients with multiple CVD risks Traditional risk factors Emerging biomarkers Multifactorial risk reduction Improved clinical outcome Clinical trials
 Activation tree example
Activation tree example Exploitation plan example
Exploitation plan example Hip fracture care clinical care standard
Hip fracture care clinical care standard Therapeutic services pathway
Therapeutic services pathway Delirium care pathways
Delirium care pathways Care pathways examples
Care pathways examples Primary secondary tertiary health care
Primary secondary tertiary health care Activation-synthesis theory
Activation-synthesis theory Overpotential
Overpotential Stage n1
Stage n1 Ares activation code
Ares activation code Activation synthesis theory
Activation synthesis theory Secondstep.org activation key
Secondstep.org activation key Activation synthesis
Activation synthesis Spreading activation psychology
Spreading activation psychology Prototype in semantics
Prototype in semantics Pupilles punctiformes
Pupilles punctiformes Mind tech international
Mind tech international Polyclonal activation
Polyclonal activation Equation d'activation et desactivation grafcet
Equation d'activation et desactivation grafcet Aglet activation code
Aglet activation code Activation energy unit
Activation energy unit