PPAR activation Clinical evidence Evolution of clinical evidence





- Slides: 18

PPAR activation Clinical evidence

Evolution of clinical evidence supporting PPAR activation Surrogate outcomes studies Large observational studies 2000 Endothelial function Carotid atherosclerosis Restenosis Ongoing clinical outcomes studies 2005 and beyond Onset of diabetes in patients with IFG Mortality in patients with diabetes + HF or AMI

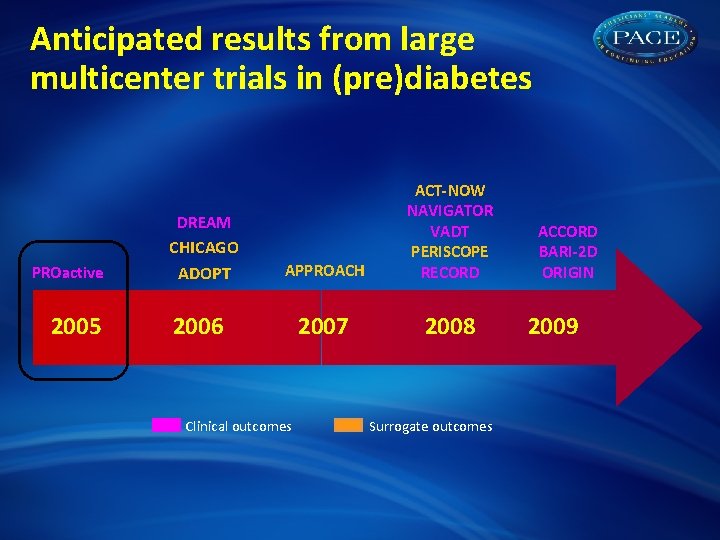
Anticipated results from large multicenter trials in (pre)diabetes APPROACH ACT-NOW NAVIGATOR VADT PERISCOPE RECORD 2007 2008 DREAM PROactive 2005 CHICAGO ADOPT 2006 Clinical outcomes Surrogate outcomes ACCORD BARI-2 D ORIGIN 2009

PROactive: Study Design PROspective pioglit. Azone Clinical Trial In macro. Vascular Events Randomized, double-blind controlled trial N = 5238 with type 2 diabetes and macrovascular disease Pioglitazone 15 mg qd titrated to 45 mg qd Placebo Primary outcome: Composite of all-cause mortality, MI (including silent MI), ACS, stroke, revascularization, leg amputation Secondary outcome: All-cause mortality, MI (excluding silent MI), stroke Mean follow-up: 34. 5 months Dormandy JA et al. Lancet. 2005; 366: 1279 -89.

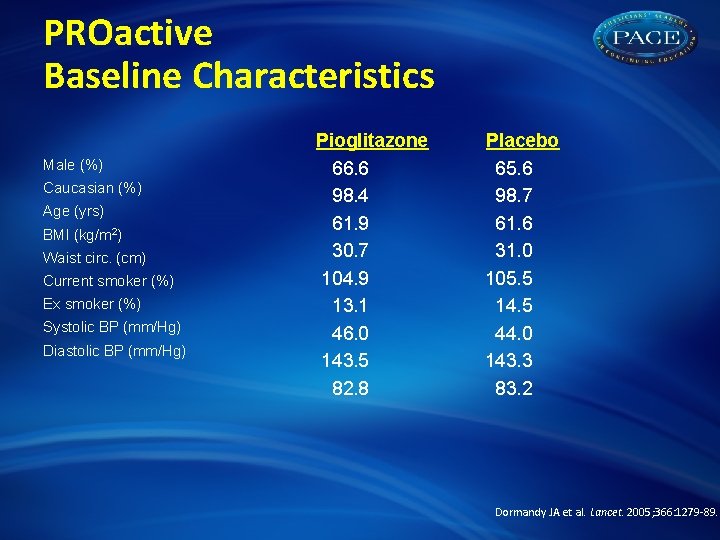
PROactive Baseline Characteristics Male (%) Caucasian (%) Age (yrs) BMI (kg/m 2) Waist circ. (cm) Current smoker (%) Ex smoker (%) Systolic BP (mm/Hg) Diastolic BP (mm/Hg) Pioglitazone 66. 6 98. 4 61. 9 30. 7 104. 9 13. 1 46. 0 143. 5 82. 8 Placebo 65. 6 98. 7 61. 6 31. 0 105. 5 14. 5 44. 0 143. 3 83. 2 Dormandy JA et al. Lancet. 2005; 366: 1279 -89.

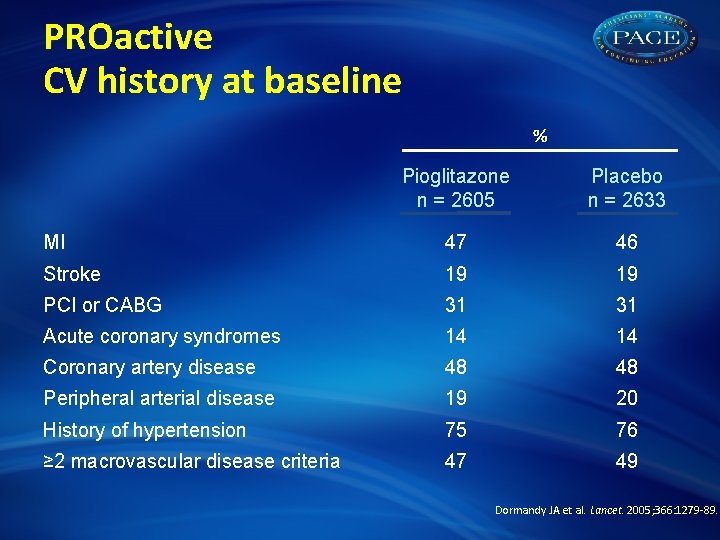
PROactive CV history at baseline % Pioglitazone n = 2605 Placebo n = 2633 MI 47 46 Stroke 19 19 PCI or CABG 31 31 Acute coronary syndromes 14 14 Coronary artery disease 48 48 Peripheral arterial disease 19 20 History of hypertension 75 76 ≥ 2 macrovascular disease criteria 47 49 Dormandy JA et al. Lancet. 2005; 366: 1279 -89.

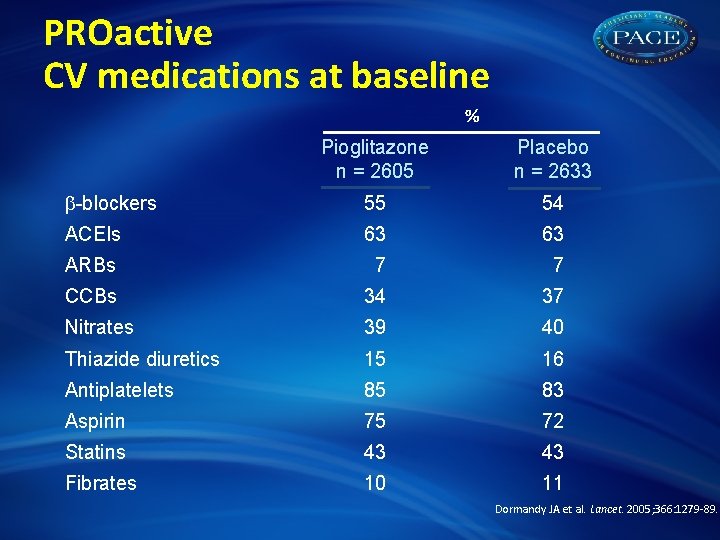
PROactive CV medications at baseline % Pioglitazone n = 2605 Placebo n = 2633 -blockers 55 54 ACEIs 63 63 ARBs 7 7 CCBs 34 37 Nitrates 39 40 Thiazide diuretics 15 16 Antiplatelets 85 83 Aspirin 75 72 Statins 43 43 Fibrates 10 11 Dormandy JA et al. Lancet. 2005; 366: 1279 -89.

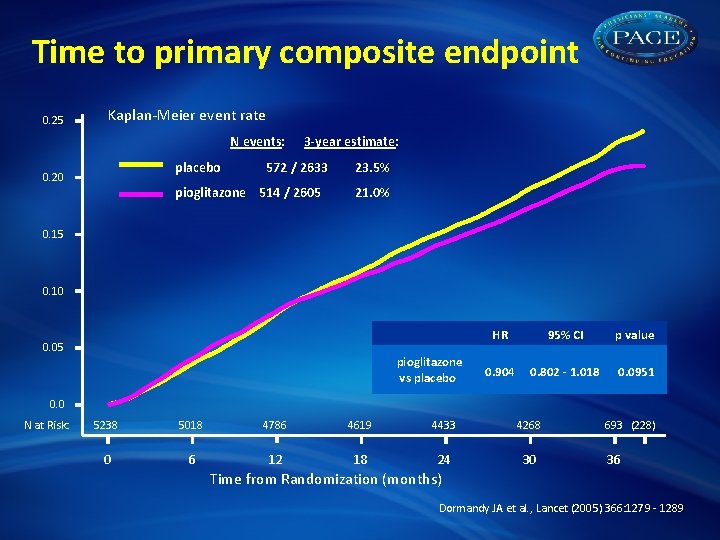
Time to primary composite endpoint 0. 25 Kaplan-Meier event rate N events: placebo 0. 20 3 -year estimate: 572 / 2633 pioglitazone 514 / 2605 23. 5% 21. 0% 0. 15 0. 10 0. 05 pioglitazone vs placebo HR 95% CI p value 0. 904 0. 802 - 1. 018 0. 0951 0. 0 N at Risk: 5238 5018 4786 4619 4433 4268 0 6 12 18 24 30 693 (228) 36 Time from Randomization (months) Dormandy JA et al. , Lancet (2005) 366: 1279 - 1289

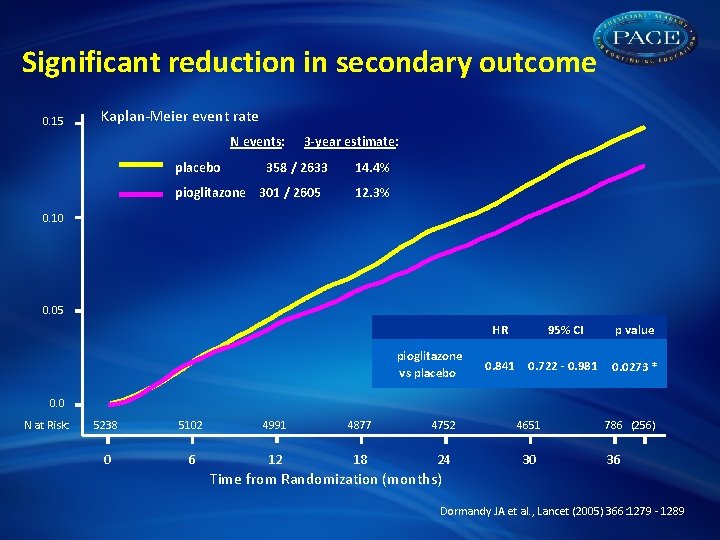
Significant reduction in secondary outcome 0. 15 Kaplan-Meier event rate N events: placebo 3 -year estimate: 358 / 2633 pioglitazone 301 / 2605 14. 4% 12. 3% 0. 10 0. 05 pioglitazone vs placebo HR 95% CI p value 0. 841 0. 722 - 0. 981 0. 0273 * 0. 0 N at Risk: 5238 5102 4991 4877 4752 4651 0 6 12 18 24 30 786 (256) 36 Time from Randomization (months) Dormandy JA et al. , Lancet (2005) 366: 1279 - 1289

Time to all-cause death, non-fatal MI, stroke or ACS 0. 20 Kaplan-Meier event rate N events: placebo 3 -year estimate: 409 / 2633 pioglitazone 339 / 2605 0. 15 16. 5% 13. 8% 0. 10 0. 05 pioglitazone vs placebo HR 95% CI p value 0. 828 0. 717 - 0. 956 0. 01 * 0. 0 N at Risk: 5238 5080 4947 4816 4684 4564 0 6 12 18 24 30 765 (248) 36 Time from Randomization (months) Dormandy JA et al. , Lancet (2005) 366: 1279 - 1289

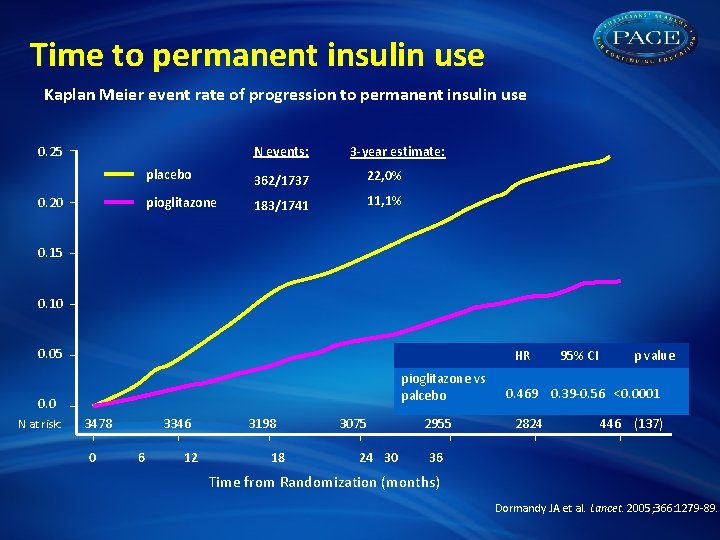
Time to permanent insulin use Kaplan Meier event rate of progression to permanent insulin use N events: 0. 25 0. 20 3 -year estimate: placebo 362/1737 22, 0% pioglitazone 183/1741 11, 1% 0. 15 0. 10 0. 05 HR pioglitazone vs palcebo 0. 0 N at risk: 3478 0 3346 6 12 3198 18 3075 24 30 2955 95% CI p value 0. 469 0. 39 -0. 56 <0. 0001 2824 446 (137) 36 Time from Randomization (months) Dormandy JA et al. Lancet. 2005; 366: 1279 -89.

PROactive Subgroup analysis – Previous MI n = 2445 with previous MI (≥ 6 mo) • Pioglitazone reduced risk of CV events, including: – Fatal/nonfatal MI* by 28% (P = 0. 045) – ACS by 37% (P = 0. 035) • Over 3 years, pioglitazone added to medication in 1000 patients could prevent: – 22 recurrent MIs – 23 ACS events • Future studies are needed to further elucidate the underlying mechanism(s) of these clinical results *Excluding silent MI Adapted from Erdmann E. AHA 2005. www. PROactive-results. com.

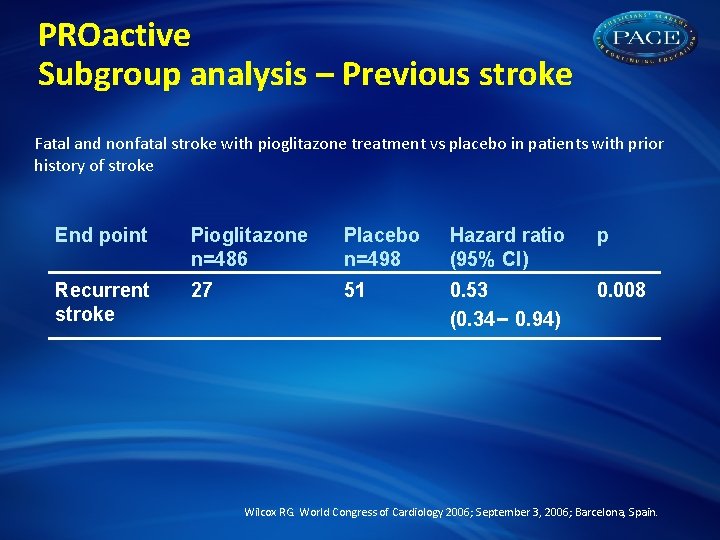
PROactive Subgroup analysis – Previous stroke Fatal and nonfatal stroke with pioglitazone treatment vs placebo in patients with prior history of stroke End point Pioglitazone n=486 Placebo n=498 Hazard ratio (95% CI) p Recurrent stroke 27 51 0. 53 (0. 34– 0. 94) 0. 008 Wilcox RG. World Congress of Cardiology 2006; September 3, 2006; Barcelona, Spain.

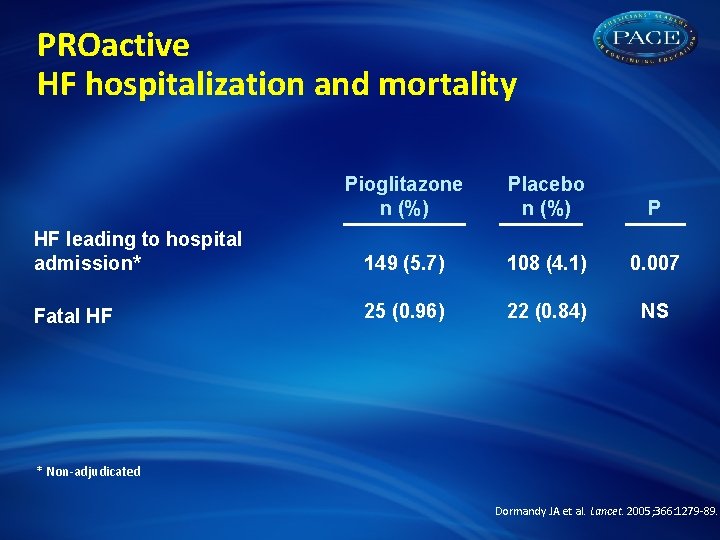
PROactive HF hospitalization and mortality Pioglitazone n (%) Placebo n (%) P HF leading to hospital admission* 149 (5. 7) 108 (4. 1) 0. 007 Fatal HF 25 (0. 96) 22 (0. 84) NS * Non-adjudicated Dormandy JA et al. Lancet. 2005; 366: 1279 -89.

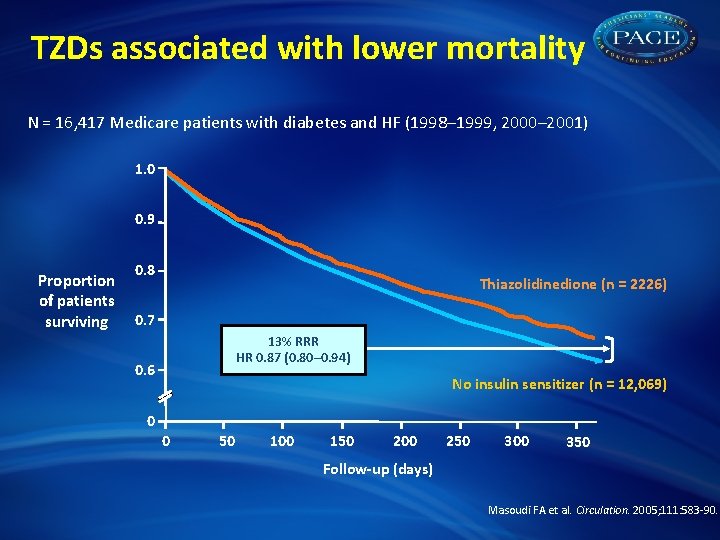
TZDs associated with lower mortality N = 16, 417 Medicare patients with diabetes and HF (1998– 1999, 2000– 2001) 1. 0 0. 9 Proportion of patients surviving 0. 8 Thiazolidinedione (n = 2226) 0. 7 13% RRR HR 0. 87 (0. 80– 0. 94) 0. 6 No insulin sensitizer (n = 12, 069) 0 0 50 100 150 200 250 300 350 Follow-up (days) Masoudi FA et al. Circulation. 2005; 111: 583 -90.

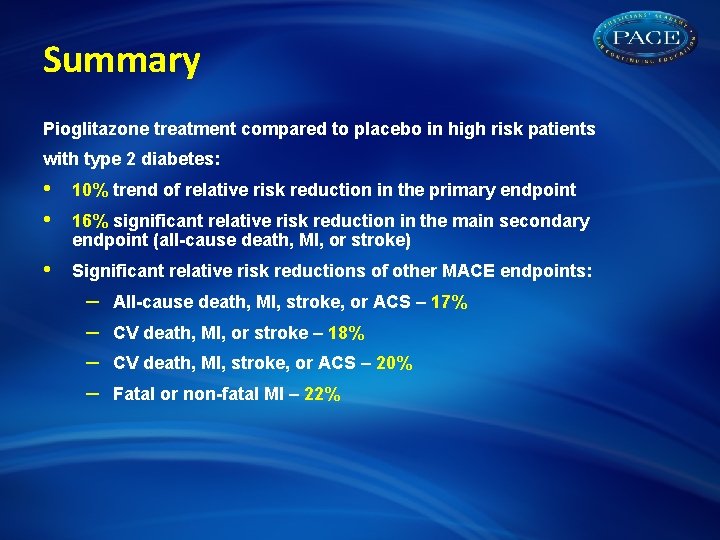
Summary Pioglitazone treatment compared to placebo in high risk patients with type 2 diabetes: • • 10% trend of relative risk reduction in the primary endpoint • Significant relative risk reductions of other MACE endpoints: 16% significant relative risk reduction in the main secondary endpoint (all-cause death, MI, or stroke) – – All-cause death, MI, stroke, or ACS – 17% CV death, MI, or stroke – 18% CV death, MI, stroke, or ACS – 20% Fatal or non-fatal MI – 22%

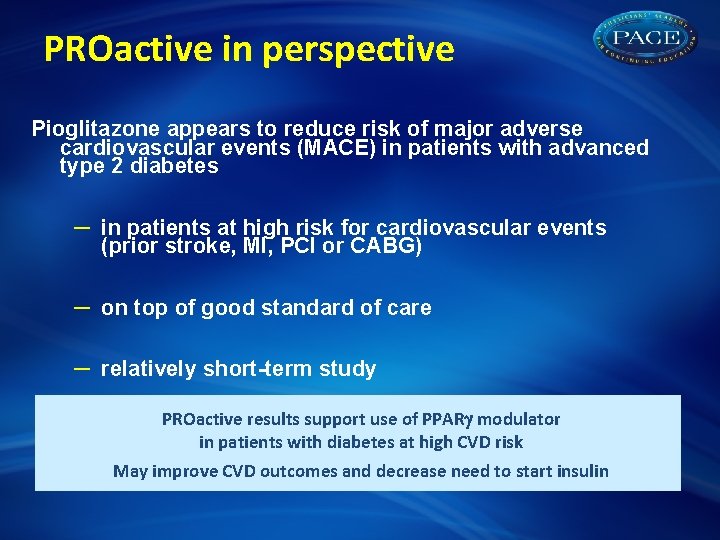
PROactive in perspective Pioglitazone appears to reduce risk of major adverse cardiovascular events (MACE) in patients with advanced type 2 diabetes – in patients at high risk for cardiovascular events (prior stroke, MI, PCI or CABG) – on top of good standard of care – relatively short-term study PROactive results support use of PPAR modulator in patients with diabetes at high CVD risk May improve CVD outcomes and decrease need to start insulin

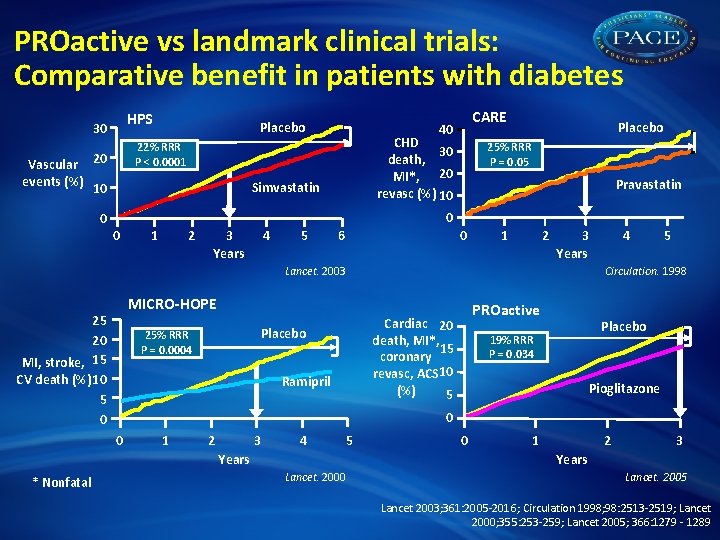
PROactive vs landmark clinical trials: Comparative benefit in patients with diabetes HPS 30 Vascular events (%) 10 CARE 40 CHD 30 death, MI*, 20 revasc (%) 10 22% RRR P < 0. 0001 20 0 Placebo Simvastatin Placebo 25% RRR P = 0. 05 Pravastatin 0 0 1 2 3 Years 4 5 0 6 1 2 3 Years MICRO-HOPE Ramipril Placebo 19% RRR P = 0. 034 Pioglitazone 0 0 1 2 3 4 5 Years * Nonfatal PROactive Cardiac 20 death, MI*, 15 coronary revasc, ACS 10 (%) 5 Placebo 25% RRR P = 0. 0004 5 Circulation. 1998 Lancet. 2003 25 20 MI, stroke, 15 CV death (%) 10 5 0 4 0 1 2 3 Years Lancet. 2000 Lancet. 2005 Lancet 2003; 361: 2005 -2016; Circulation 1998; 98: 2513 -2519; Lancet 2000; 355: 253 -259; Lancet 2005; 366: 1279 - 1289
 Activation tree and activation record
Activation tree and activation record Embryology provides evidence for evolution because
Embryology provides evidence for evolution because Embryology evidence of evolution
Embryology evidence of evolution 4 types of evidence for evolution
4 types of evidence for evolution Evidence for evolution
Evidence for evolution Examples of fossil evidence
Examples of fossil evidence 5 evidence of evolution
5 evidence of evolution Tiger adaptations
Tiger adaptations Charles darwin
Charles darwin What are the four main lines of evidence for evolution?
What are the four main lines of evidence for evolution? Evidence of evolution
Evidence of evolution Indirect evidence of evolution
Indirect evidence of evolution Evidence of evolution stations
Evidence of evolution stations Evolution berkeley
Evolution berkeley Convergent evolution definition
Convergent evolution definition Biological evidence of evolution
Biological evidence of evolution What are the four types of evidence for evolution
What are the four types of evidence for evolution Evidence for evolution doodle notes
Evidence for evolution doodle notes Evidence of evolution of remnants and impressions *
Evidence of evolution of remnants and impressions *