POCT Quality Control Introduction Pointofcare testing POCT is



- Slides: 29

POCT Quality Control

Introduction Point-of-care testing (POCT) is a method of clinical laboratory testing in which the analysis is performed at the site close to where healthcare is provided to the patient. Other terms used to describe POCT have included: bedside, near patient, physician’s office, extra- laboratory, . . 2

Introduction POCT is an increasingly popular means of delivering laboratory testing. When used appropriately, POCT has clearly been shown to improve patient outcome by providing a faster result with faster therapeutic intervention. However, when incorrectly performed, POCT presents a significant patient risk and potential for increased healthcare costs. 3

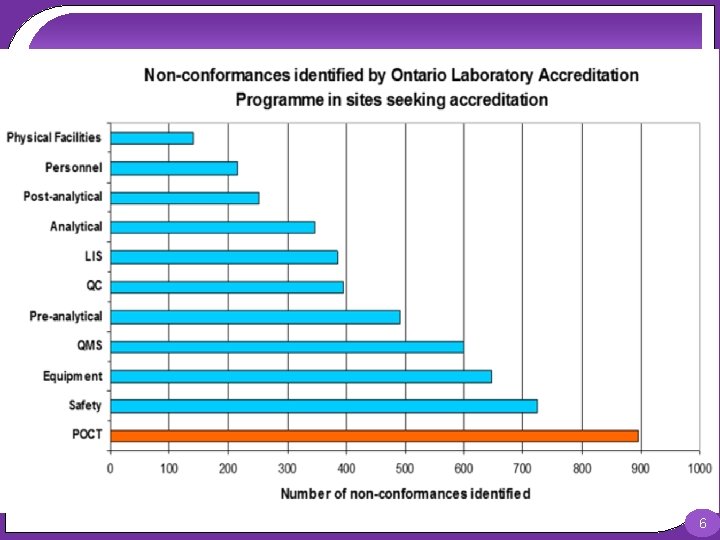
Stability of POCT Procedures POCT procedures have been previously shown to be less stable than those run within the laboratory. A study undertaken by the Ontario Laboratory Accreditation body found that POCT is the largest source of error in the laboratory when compared to other sources of error. This demonstrates the definite need for a well-designed QC procedure in a point-of-care (POC) setting to minimize the risk associated with POCT devices. 4

Stability of POCT Procedures Furthermore, another recent study found that the most common phase for errors in POCT was analytical, with 65. 3% of errors occurring during this phase. Conversely, in laboratory based testing the analytical phase is the least common source for errors. Therefore, highlighting the importance of QC procedures for POCT devices while also outlining how the potential risk of harm to a patient may be greater for POC tests compared to those performed on laboratory based analysers. 5

6

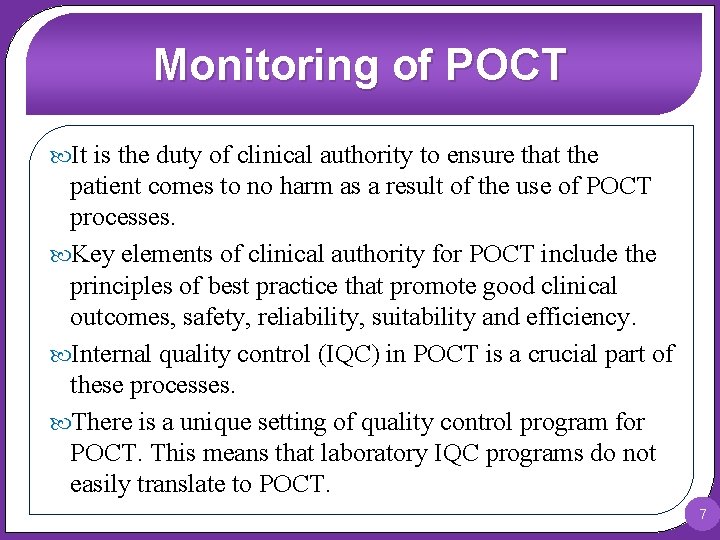
Monitoring of POCT It is the duty of clinical authority to ensure that the patient comes to no harm as a result of the use of POCT processes. Key elements of clinical authority for POCT include the principles of best practice that promote good clinical outcomes, safety, reliability, suitability and efficiency. Internal quality control (IQC) in POCT is a crucial part of these processes. There is a unique setting of quality control program for POCT. This means that laboratory IQC programs do not easily translate to POCT. 7

Monitoring of POCT ISO Standard “ 22870: 2016” ISO 22870: 2016 advises that where available, IQC and participation in an External Quality Assessment (EQA) scheme is required in the POC setting. The ISO standard that relates to POCT is ISO 22870: 2016 POCT - Requirements for quality and competence. This standard gives specific requirements applicable to POCT testing and apply when POCT is carried out in a hospital, clinic or a healthcare organization providing ambulatory care. However, it is important to note that ISO 22870 is not to be seen as a separate, standalone document, in fact, it is to be used in conjunction with ISO 15189: 2012 (medical laboratories – particular requirements for quality & competence). 8

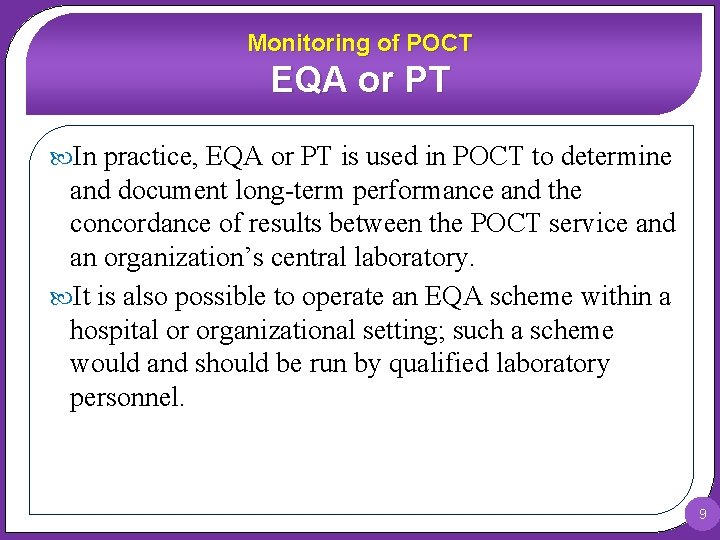
Monitoring of POCT EQA or PT In practice, EQA or PT is used in POCT to determine and document long-term performance and the concordance of results between the POCT service and an organization’s central laboratory. It is also possible to operate an EQA scheme within a hospital or organizational setting; such a scheme would and should be run by qualified laboratory personnel. 9

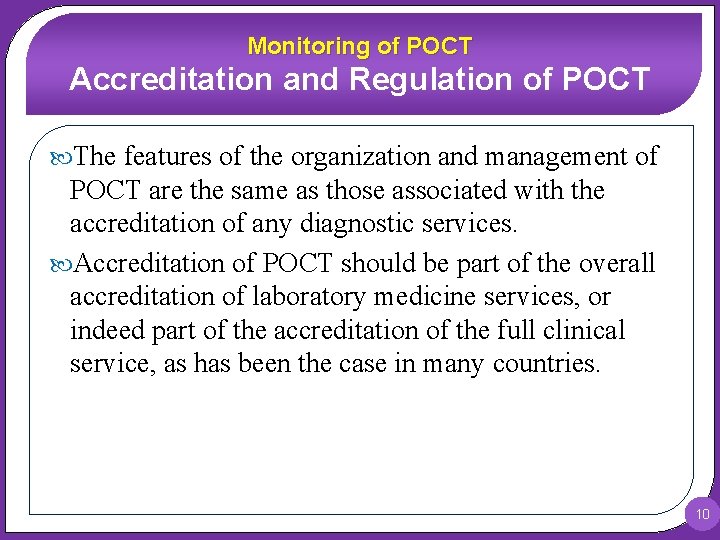
Monitoring of POCT Accreditation and Regulation of POCT The features of the organization and management of POCT are the same as those associated with the accreditation of any diagnostic services. Accreditation of POCT should be part of the overall accreditation of laboratory medicine services, or indeed part of the accreditation of the full clinical service, as has been the case in many countries. 10

Who is Responsible for QC in POCT Testing? According to ISO 22870: 2016 a POCT management group should be set up with responsibility for managing and training staff using the equipment. This group should be responsible for the quality management strategy and implementation of a staff training program which includes quality control, for all personnel performing POCT and interpreting results from a POCT device. Additionally, running of QC samples on POCT devices should be performed by those who are using the devices regularly QC samples should be run as if they were a patient sample 11

Conduct of IQC All POCT devices, from disposable pregnancy test kits to laboratory-type blood gas analysers, should have a defined IQC protocol. Common to all devices is the requirement to conduct IQC when: 1. A new lot or new delivery of consumables is received; 2. A patient result is queried; 3. After maintenance procedures and 4. If the user suspects there has been a physical insult to the device (e. g. exposure to high temperatures or if the device is dropped). 12

Conduct of IQC The frequency of further IQC testing will be analyser- dependent and should take into account the following: Analyser complexity and availability of inbuilt system checks. Frequency of patient testing. Risk associated with release of an incorrect patient result. The number of users (and maintenance of competency). The added risk of a missed IQC. 13

Type of POCT Device and IQC Requirement Disposable kits, strip technology e. g. pregnancy test kits As a minimum requirement, strip-based devices should be subject to IQC testing at least monthly unless the manufacturer suggests more frequent analysis. Two levels of IQC, containing (1) normal and (2) clinically relevant abnormal concentrations, should be tested on a representative sample of kits (e. g. two from each testing site). Such IQC provides assurance that functionality is not affected by environmental factors to which all kits have been exposed and also assesses operator competence. Inbuilt controls, for example of pregnancy test strips, provide additional assurance of the correct function of individual kits 14

Type of POCT Device and IQC Requirement Strip-based system with automatic reader e. g. glucose meters IQC assesses the function of both the reader and disposable strip components of devices such as glucose meters. Strip-based devices generally lack an inbuilt electronic check that assesses the functionality of the reader in isolation of liquid IQC. Regular IQC is therefore required to limit the potential for a faulty reader to generate poor results for weeks before identification. It is suggested that IQC analysis is conducted every day of device use. 15

Type of POCT Device and IQC Requirement In areas of low use, IQC should be conducted weekly even when patient samples are not processed to identify faults. Such regular testing of IQC material also maintains user competence. 16

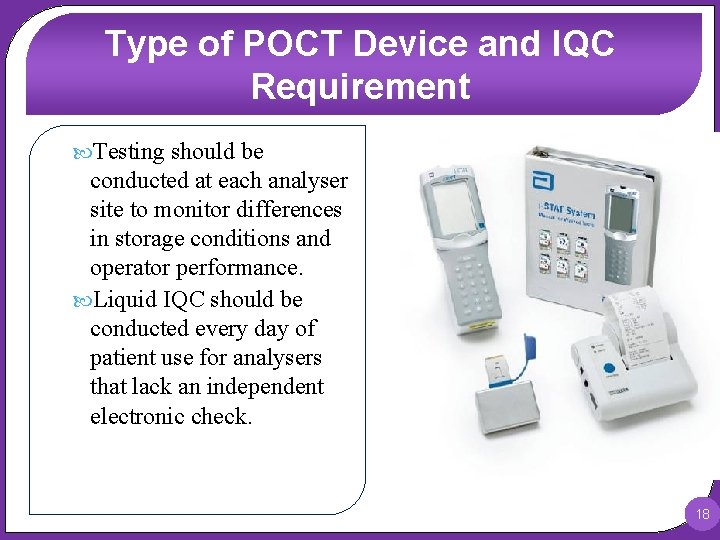
Type of POCT Device and IQC Requirement Single-use cartridge systems e. g. Abbott i-STAT and Siemens DCA Vantage Single-use cartridge systems typically employ a combination of liquid IQC with electronic IQC to assess the functionality of the whole device. Electronic IQC uses a simulator in place of the cartridge to assess the function of the reader and should be performed each day of patient use. Liquid IQC is essential to assess the analytical component (the disposable cartridge), and two levels of IQC should be tested at least monthly. 17

Type of POCT Device and IQC Requirement Testing should be conducted at each analyser site to monitor differences in storage conditions and operator performance. Liquid IQC should be conducted every day of patient use for analysers that lack an independent electronic check. 18

Type of POCT Device and IQC Requirement Multiple-use cartridge systems e. g. blood gas analysers Multi-use cartridge technology forms the basis of many multi-analyte instruments. These more complex analysers require daily multilevel IQC, equivalent to laboratory based systems. However, the conduct of IQC is user independent for latest generation analysers. Rather, IQC is run automatically at predefined time points and assesses the fluidics path of a patient sample as well as the measurement system. 19

Type of POCT Device and IQC Requirement Some systems include continuous integrated IQC which checks the quality of the signal, and sample integrity, every time a patient sample is run. Unfortunately, manually processed IQC is also limited in this respect if the material does not mimic patient specimens. 20

IQC Material In contrast to the laboratory setting, there is a reliance on the manufacturer both to provide material and to assign acceptable ranges for IQC in POCT. Indeed, some manufacturers state that their own IQC material should be used rather than third-party material. However, it is recommended that third-party IQC material is used to provide an independent check of the whole testing system where possible. 21

IQC Material IQC material should contain analytes at clinically relevant concentrations, guided by the clinical setting of the POCT instrument. For example, IQC materials containing higher levels of bilirubin may be necessary for neonatal units compared to other clinical settings. The material should be provided ready for use – with any manipulation of the material (e. g. reconstitution) completed prior to distribution. It should also ideally be stable at ambient temperature to minimize storage problems. 22

Documentation and review of IQC results One of the major challenges in POCT is achieving proper documentation. All IQC values obtained by POCT must be recorded along with the date/time, user ID and consumable lot numbers. The user decision to accept/reject IQC, plus actions as appropriate, must also be documented. 23

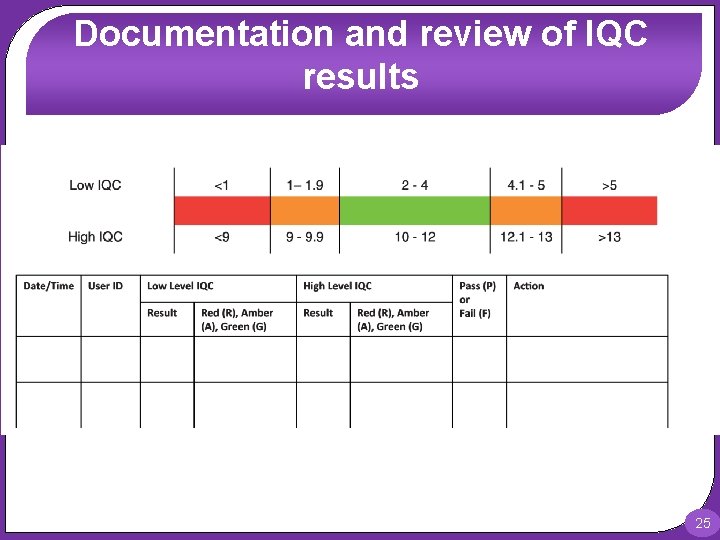
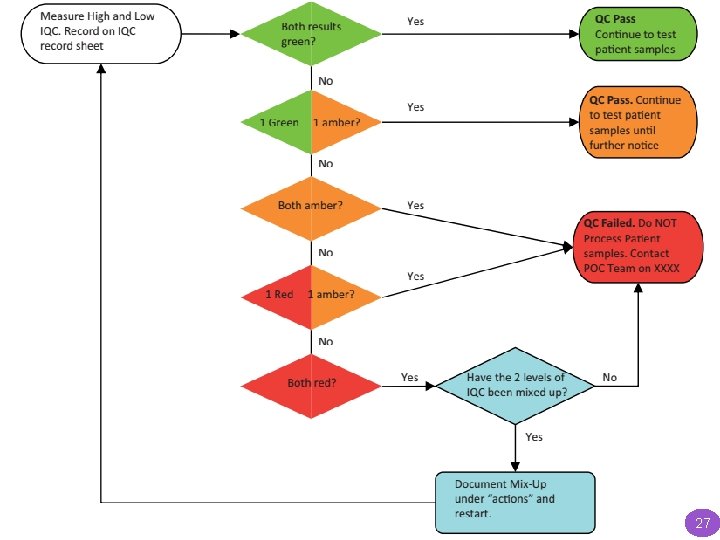
Documentation and review of IQC results For quantitative tests requiring manual recording, a two-tier acceptance/rejection criteria would improve error detection rates. A flow chart based on the system used within the Australian Government’s point of care testing in general practice trial is provided in the Figure. This system classifies the results of high and low IQC as: Green (within 2 SD of mean), Amber (between 2 and 3 SD) or red (greater than 3 SD). The combination of the high and low QC color code is then used to decide if the QC has passed or failed. 24

Documentation and review of IQC results 25

Documentation and review of IQC results ‘Red’ results should immediately initiate review by the POCT team via an alert from the end user, while the number of green/amber results should be reviewed by the POCT team on a regular basis, e. g. monthly. Standardized flow charts plus IQC documentation forms, such as those shown in the Figure, could be used across analysers to aid correct interpretation and initiate appropriate action. 26

27

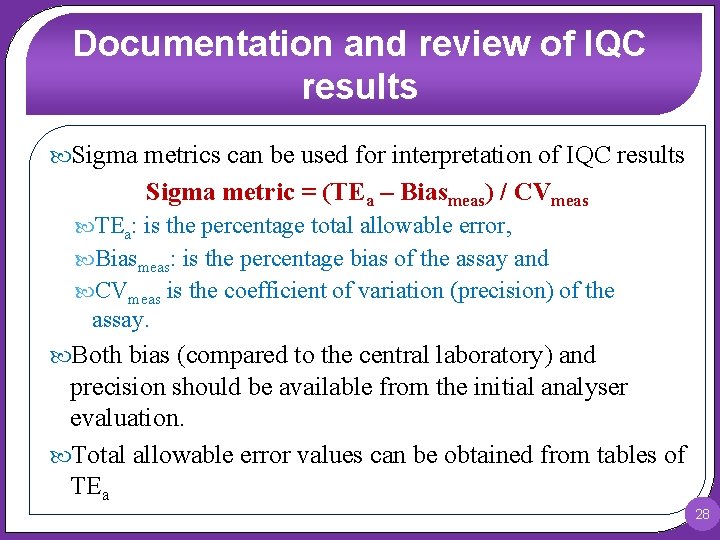
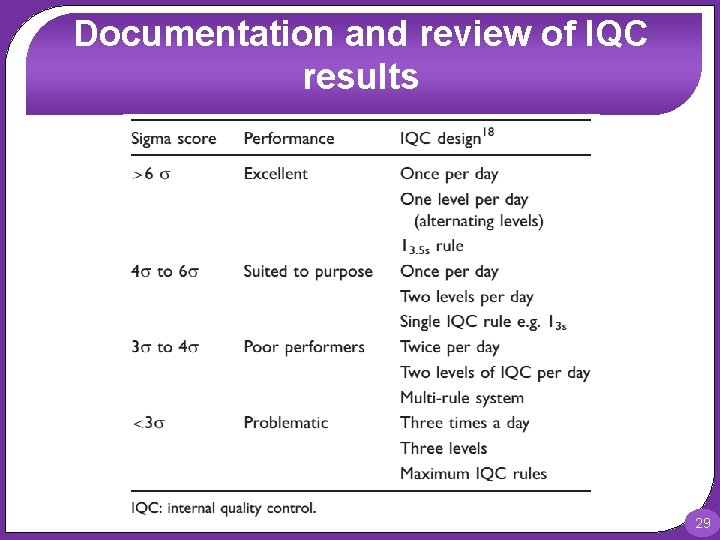
Documentation and review of IQC results Sigma metrics can be used for interpretation of IQC results Sigma metric = (TEa – Biasmeas) / CVmeas TEa: is the percentage total allowable error, Biasmeas: is the percentage bias of the assay and CVmeas is the coefficient of variation (precision) of the assay. Both bias (compared to the central laboratory) and precision should be available from the initial analyser evaluation. Total allowable error values can be obtained from tables of TEa 28

Documentation and review of IQC results 29