PID Evaluation and Clinical Health PEACH Study PEACH




















- Slides: 30

PID Evaluation and Clinical Health (PEACH) Study

PEACH Multi-center randomized clinical trial of 831 women followed 2 -5 yrs through 10/00

Study treatment • Inpatient 2 gr Cefoxitin IV every 6 hours • Outpatient 2 gr Cefoxitin 1 gr Probenecid 1 shot 100 grs Doxiciclyne IV 100 mg Doxiciclyne oral twice a day Every 12 hours

Eligibility of patients into the study Inclusion (Must meet all of the following criteria) 1. 37 years of age or younger 2. Willing to participate 3. Presenting with a history of pelvic discomfort for <30 days (this does not need to be the chief complain) 4. Experiencing pelvic organ tenderness (uterine or adnexal tenderness) on bilateral examination 5. Leukorrhea and/or Mucopurulent Cervicitis and/or untreated known + GC or CT

Eligibility of patients into the study Exclusion (Must not meet any of the following criteria) 1. Currently pregnant by urine testing. 2. Tubo-ovarian abscess. 3. Appendicitis, hemorrhagic ovarian cyst or other condition requiring surgery by ultrasound or laparoscopy. 4. Nausea or vomiting after a trial of metocopramide 5. Antimicrobial therapy within the past 7 days

Eligibility of patients into the study Exclusion 6. Delivery, abortion or gynecology surgery with the past 30 days. 7. Prior hysterectomy or bilateral salipingectomy. 8. Allergy to penicillins, cephalosporins or tetracyclines. 9. Homeless.


Outcomes Primary Involuntary infertility

Others Outcomes Short term: • Time to clinical improvement • Microbiologic cure on repeat cervical culture and endometrial biopsy • Patient satisfaction with medical care • Treatment adherence

Others Outcomes Long term: • Tubal occlusion in women with involuntary infertility • Repeat episodes of PID • Ectopic pregnancy • Functional decline due to pelvic pain • Quality of life • Frequency of health service use and indirect PID-related cost, cost-utility analysis

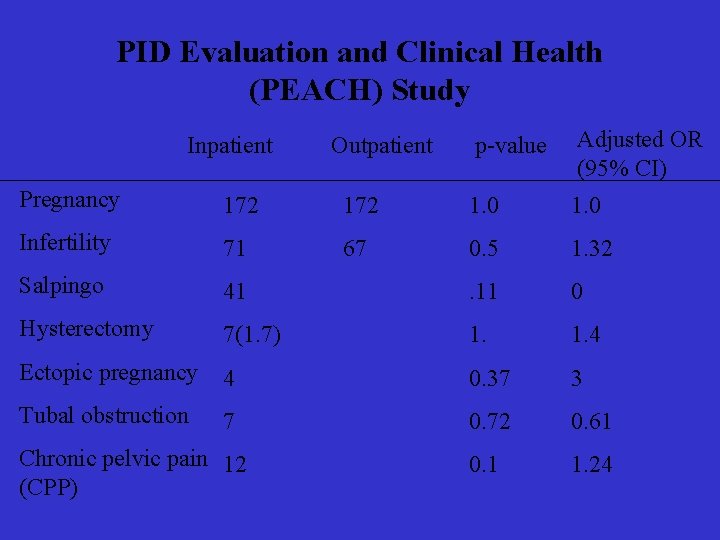
PID Evaluation and Clinical Health (PEACH) Study Inpatient Outpatient p-value Adjusted OR (95% CI) Pregnancy 172 1. 0 Infertility 71 67 0. 5 1. 32 Salpingo 41 . 11 0 Hysterectomy 7(1. 7) 1. 4 Ectopic pregnancy 4 0. 37 3 Tubal obstruction 7 0. 72 0. 61 0. 1 1. 24 Chronic pelvic pain 12 (CPP)

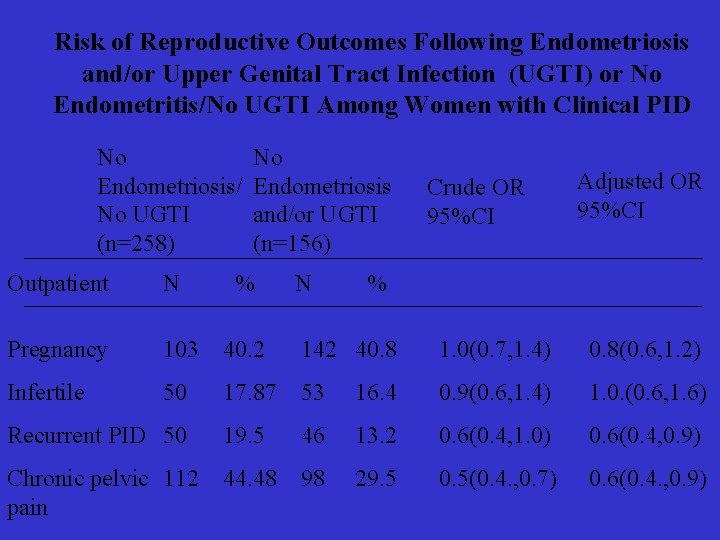
Risk of Reproductive Outcomes Following Endometriosis and/or Upper Genital Tract Infection (UGTI) or No Endometritis/No UGTI Among Women with Clinical PID No Endometriosis/ No UGTI (n=258) No Endometriosis and/or UGTI (n=156) % N Crude OR 95%CI Adjusted OR 95%CI Outpatient N % Pregnancy 103 40. 2 142 40. 8 1. 0(0. 7, 1. 4) 0. 8(0. 6, 1. 2) Infertile 50 17. 87 53 16. 4 0. 9(0. 6, 1. 4) 1. 0. (0. 6, 1. 6) Recurrent PID 50 19. 5 46 13. 2 0. 6(0. 4, 1. 0) 0. 6(0. 4, 0. 9) Chronic pelvic 112 pain 44. 48 98 29. 5 0. 5(0. 4. , 0. 7) 0. 6(0. 4. , 0. 9)

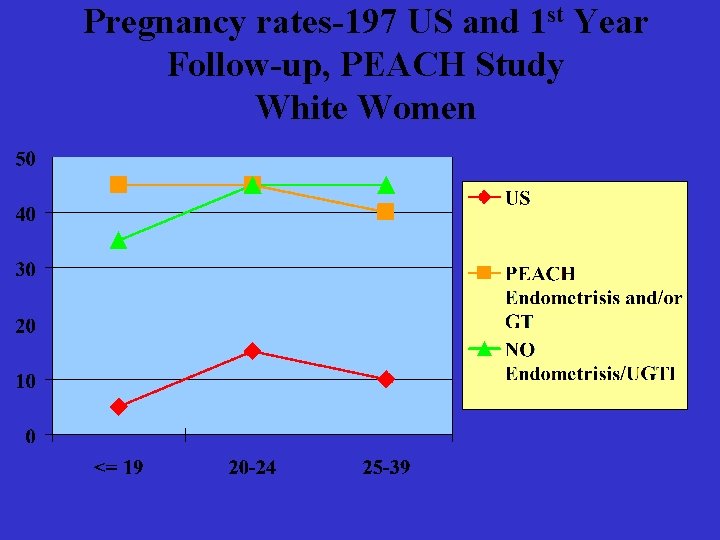
Pregnancy rates-197 US and 1 st Year Follow-up, PEACH Study White Women

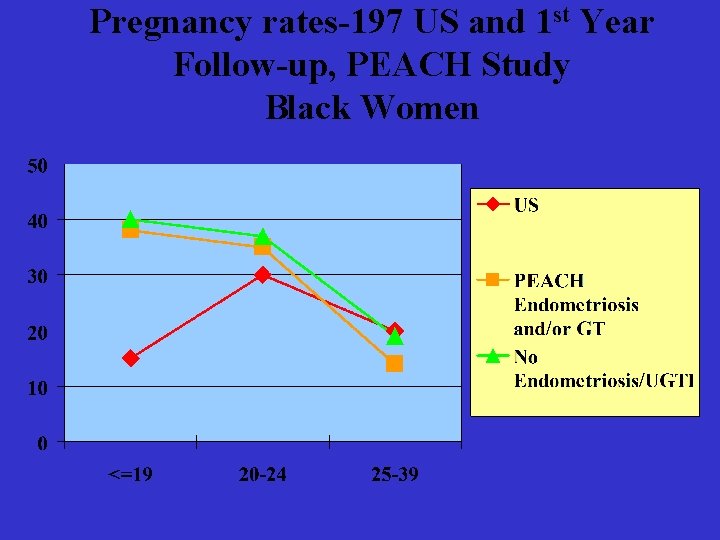
Pregnancy rates-197 US and 1 st Year Follow-up, PEACH Study Black Women

PEACH 2 Specific Aims: • To continue the follow-up of women in the PEACH study go as to better describe the rates of inability to achieve pregnancy, chronic pelvic pain, and recurrence after mild-to-moderate PID • To develop and validate a clinical prediction rule(s) that identifies women after diagnosis of PID who are at high risk for: inability to achieve pregnancy, having chronic pelvic pain, or having recurrent disease • To identify whether inpatient treatment is more effective than outpatient treatment in reducing the risk of sequelae among prognostic subset of women

DAISY Project

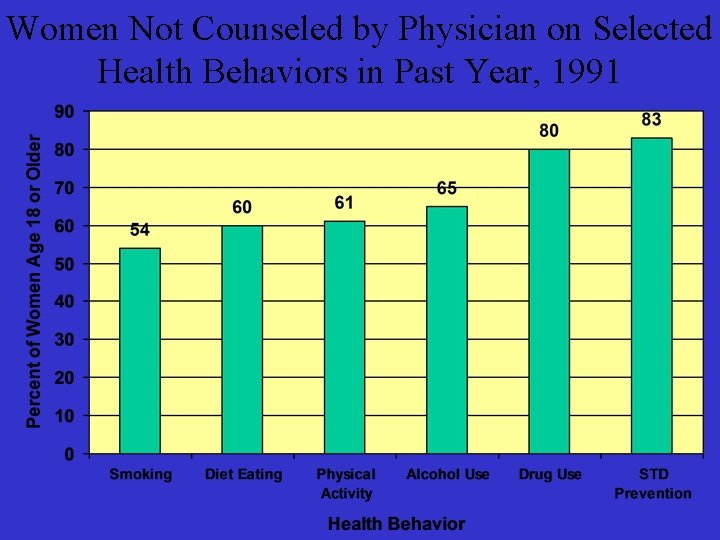
Women Not Counseled by Physician on Selected Health Behaviors in Past Year, 1991

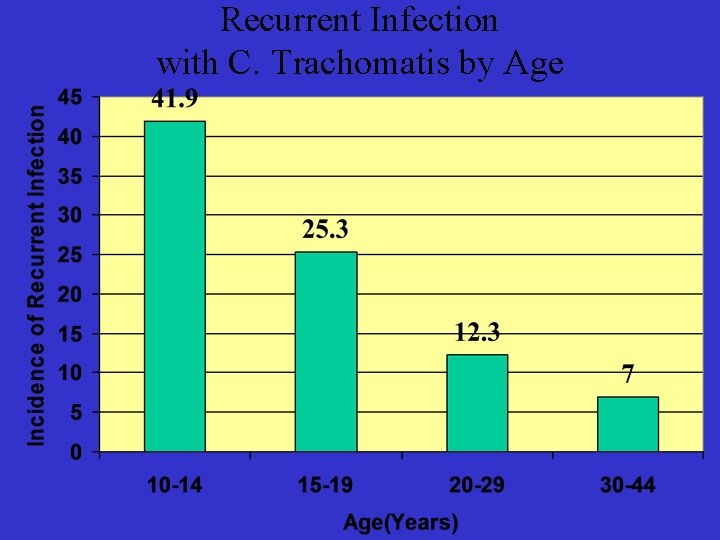
Recurrent Infection with C. Trachomatis by Age

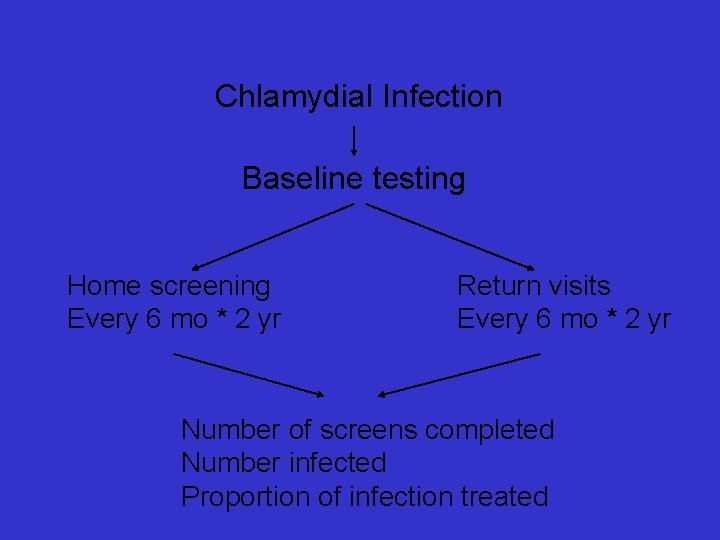
Chlamydial Infection Baseline testing Home screening Every 6 mo * 2 yr Return visits Every 6 mo * 2 yr Number of screens completed Number infected Proportion of infection treated

Hormonal Contraception and the Recognition of Pelvic Inflammatory Disease • • • RB Ness, LM Keder, DE Soper, AJ Amertegul, J Glack, H Wiesenfeld, PA Rice, JF Pelpert, A Karmbour-Shakir, SP Donegan

Epidemiologic Data • Oral Contraceptive use associated with 2* Increase prevalence of C. trachomatis. • Oral contraceptive associated with protection against symptomatic PID. • Salpingitis more mild among women using oral contraceptives.

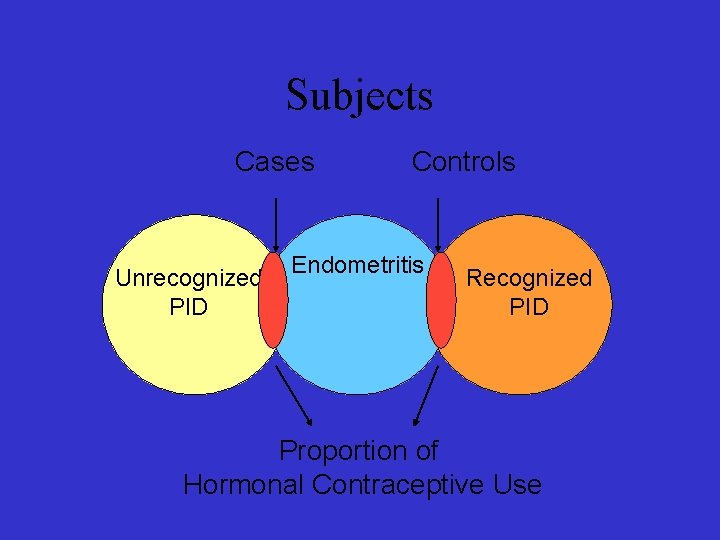
Subjects Cases Unrecognized PID Controls Endometritis Recognized PID Proportion of Hormonal Contraceptive Use

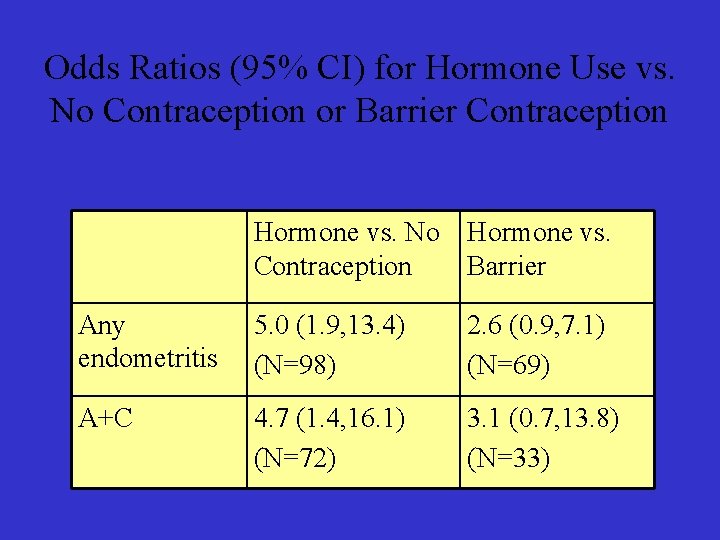
Odds Ratios (95% CI) for Hormone Use vs. No Contraception or Barrier Contraception Hormone vs. No Hormone vs. Contraception Barrier Any endometritis 5. 0 (1. 9, 13. 4) (N=98) 2. 6 (0. 9, 7. 1) (N=69) A+C 4. 7 (1. 4, 16. 1) (N=72) 3. 1 (0. 7, 13. 8) (N=33)

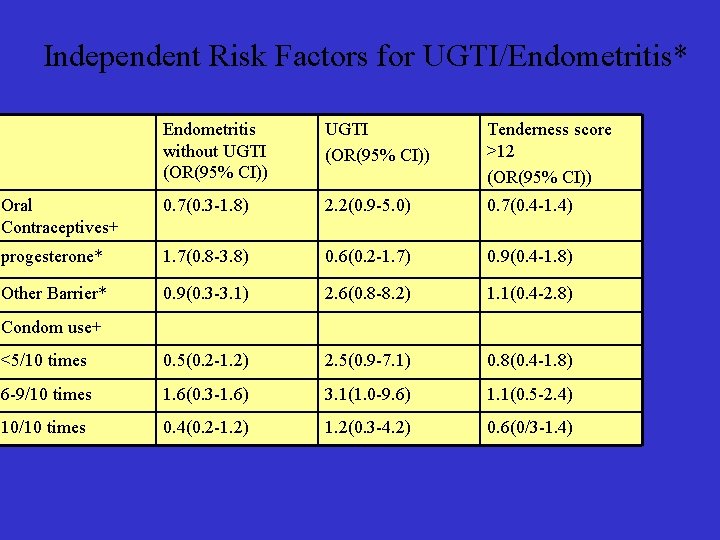
Independent Risk Factors for UGTI/Endometritis* Endometritis without UGTI (OR(95% CI)) Tenderness score >12 (OR(95% CI)) Oral Contraceptives+ 0. 7(0. 3 -1. 8) 2. 2(0. 9 -5. 0) 0. 7(0. 4 -1. 4) progesterone* 1. 7(0. 8 -3. 8) 0. 6(0. 2 -1. 7) 0. 9(0. 4 -1. 8) Other Barrier* 0. 9(0. 3 -3. 1) 2. 6(0. 8 -8. 2) 1. 1(0. 4 -2. 8) <5/10 times 0. 5(0. 2 -1. 2) 2. 5(0. 9 -7. 1) 0. 8(0. 4 -1. 8) 6 -9/10 times 1. 6(0. 3 -1. 6) 3. 1(1. 0 -9. 6) 1. 1(0. 5 -2. 4) 10/10 times 0. 4(0. 2 -1. 2) 1. 2(0. 3 -4. 2) 0. 6(0/3 -1. 4) Condom use+

Gyn Infections Follow-Through (GIFT) Study

Douching Prevalence

Bacterial Vaginosis (BV): What is it? • A replacement of the normal vaginal flora (Lactobacillus) with a mixed flora of Gardnerella vaginalis, anaerobes, and Mycoplasma hominis

Prospective Observational Study 1200 women enrolled from 5 clinical sites over 18 months

Primary Objective To compare the time to PID between women who are douching (at least once / month on average) vs. women who are not.

Prevention, prevention and prevention • Douching cessation programs • Self-testing for STDs • Predictors of infertility among women with PID • Will treating BV prevent PID?
 Multi loop pid controller regolatore pid multi loop
Multi loop pid controller regolatore pid multi loop Peach pid
Peach pid Celf 5 record form
Celf 5 record form Administrative healthcare information systems
Administrative healthcare information systems “control de grua” and posicionamiento
“control de grua” and posicionamiento James and the giant peach vocabulary by chapter
James and the giant peach vocabulary by chapter James and the giant peach vocabulary
James and the giant peach vocabulary James and the giant peach book summary
James and the giant peach book summary James and the giant peach vocabulary
James and the giant peach vocabulary The landlady setting
The landlady setting Unfolding clinical reasoning case study
Unfolding clinical reasoning case study Iwr clinical trial
Iwr clinical trial Measurement and evaluation for health educators
Measurement and evaluation for health educators Whole health clinical group
Whole health clinical group Pid meter werking
Pid meter werking Perturbaciones
Perturbaciones Lego spike line follower
Lego spike line follower Open office
Open office Pid symbols
Pid symbols Battery limit p&id
Battery limit p&id Pid rise time
Pid rise time Pid persistent identifier
Pid persistent identifier Modern control
Modern control Pid schéma
Pid schéma Hlyniany
Hlyniany Ert controller
Ert controller Pid rise time
Pid rise time Control pid
Control pid Calculo pid
Calculo pid Classical pid control
Classical pid control Pid 3 step
Pid 3 step




















































