PHYS 172 Modern Mechanics Lecture 25 Kinetic theory

- Slides: 19

PHYS 172: Modern Mechanics Lecture 25 - Kinetic theory of gases; pressure Summer 2012 Read 12. 8

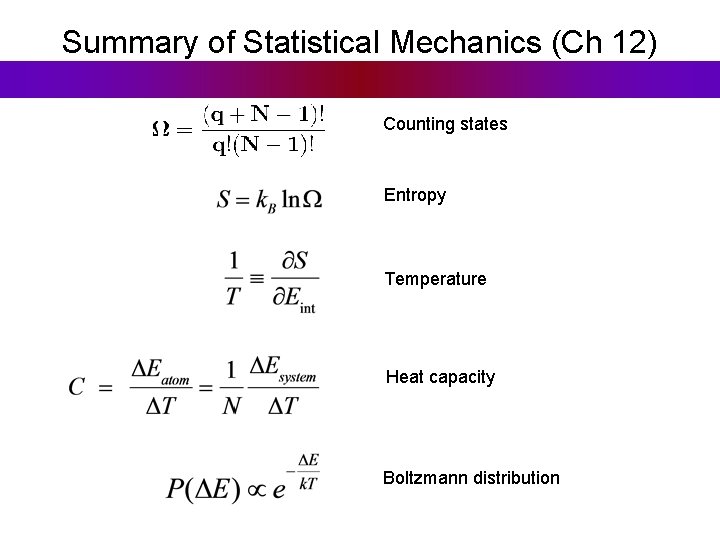
Summary of Statistical Mechanics (Ch 12) Counting states Entropy Temperature Heat capacity Boltzmann distribution

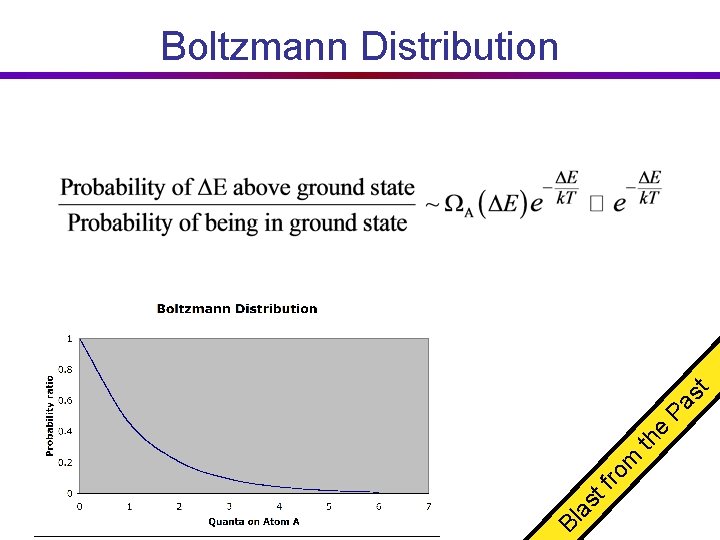
Bl as tf ro m th e Pa st Boltzmann Distribution

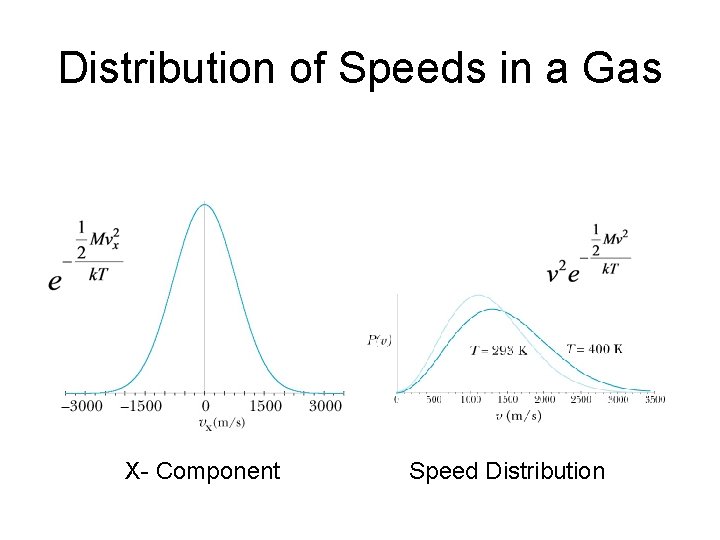
Distribution of Speeds in a Gas X- Component Speed Distribution

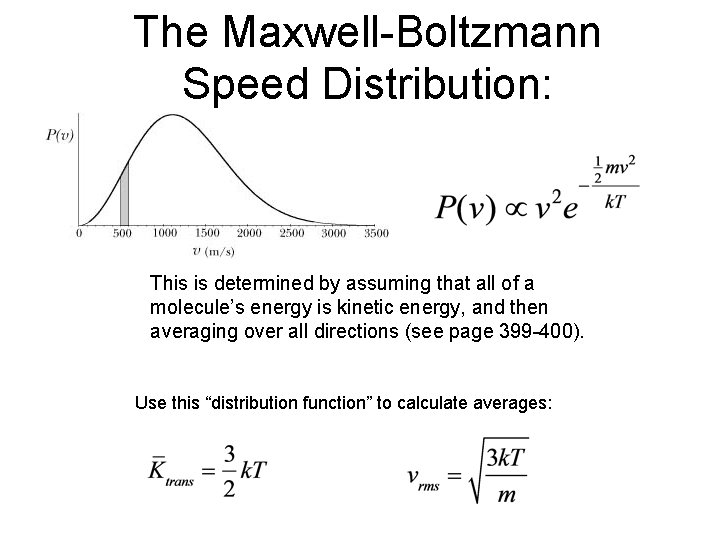
The Maxwell-Boltzmann Speed Distribution: This is determined by assuming that all of a molecule’s energy is kinetic energy, and then averaging over all directions (see page 399 -400). Use this “distribution function” to calculate averages:

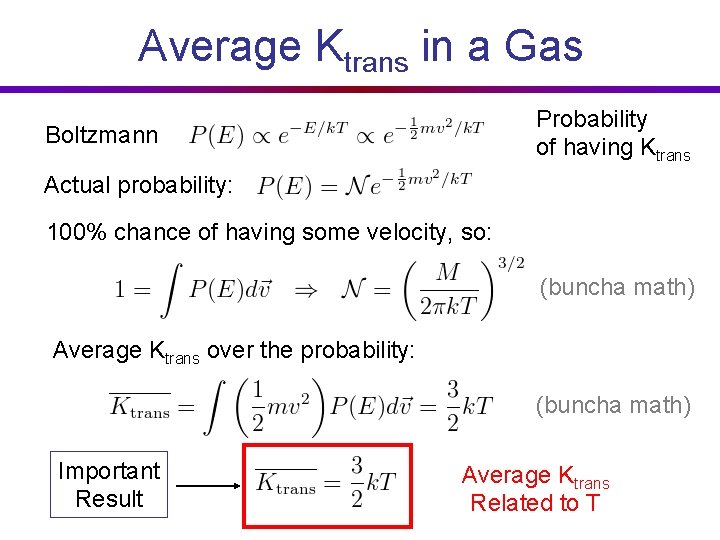
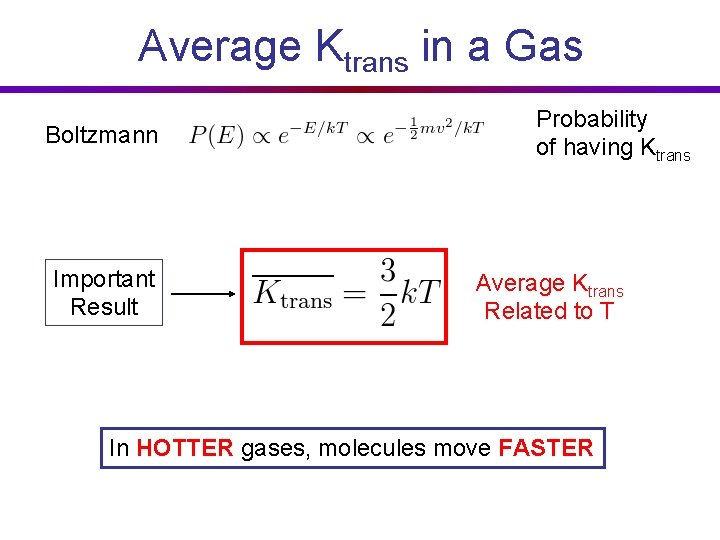
Average Ktrans in a Gas Probability of having Ktrans Boltzmann Actual probability: 100% chance of having some velocity, so: (buncha math) Average Ktrans over the probability: (buncha math) Important Result Average Ktrans Related to T

Average Ktrans in a Gas Boltzmann Important Result Probability of having Ktrans Average Ktrans Related to T In HOTTER gases, molecules move FASTER

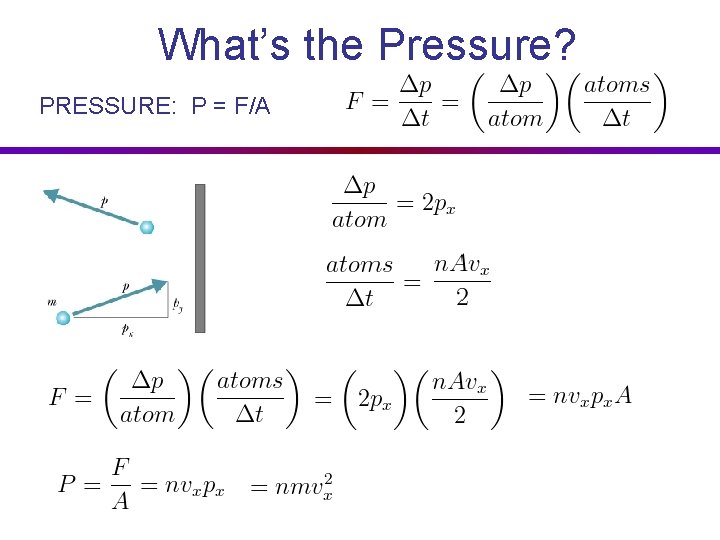
What’s the Pressure? Big Box of Gas

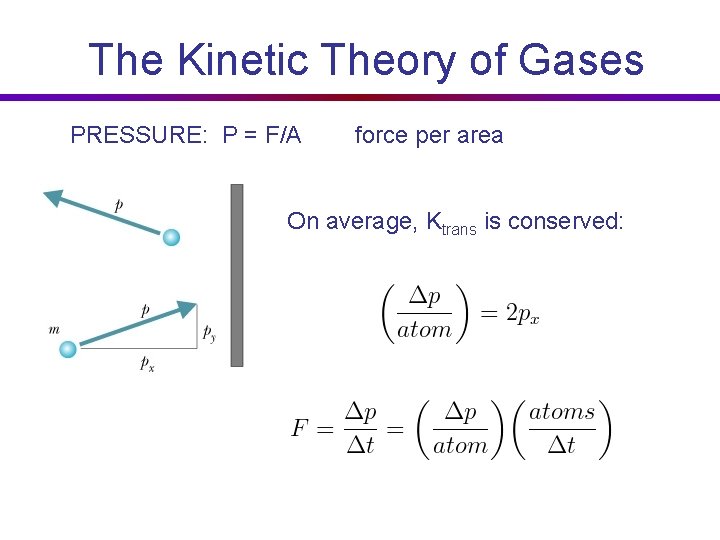
The Kinetic Theory of Gases PRESSURE: P = F/A force per area On average, Ktrans is conserved:

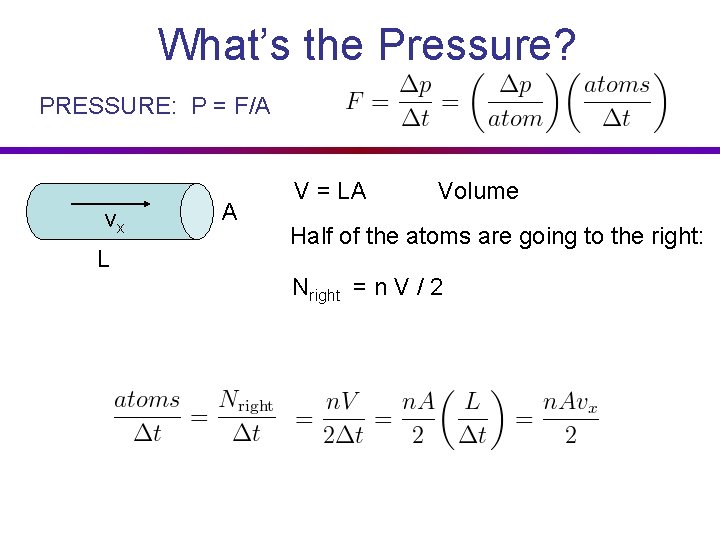
What’s the Pressure? PRESSURE: P = F/A vx L A V = LA Volume Half of the atoms are going to the right: Nright = n V / 2

What’s the Pressure? PRESSURE: P = F/A

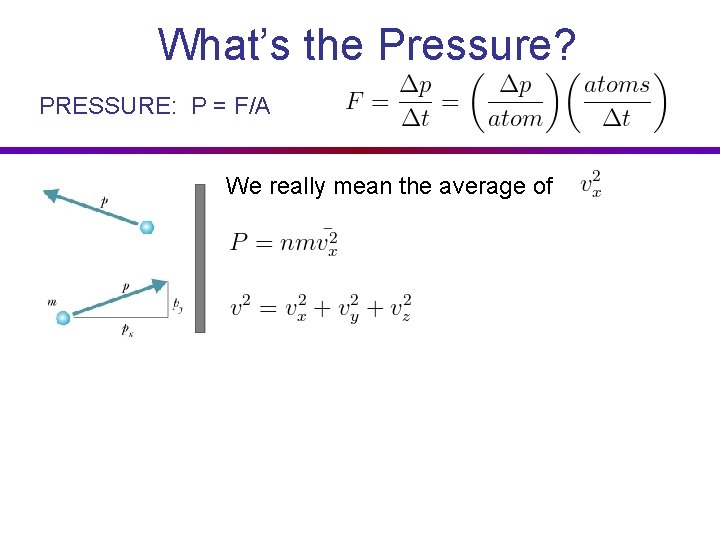
What’s the Pressure? PRESSURE: P = F/A We really mean the average of

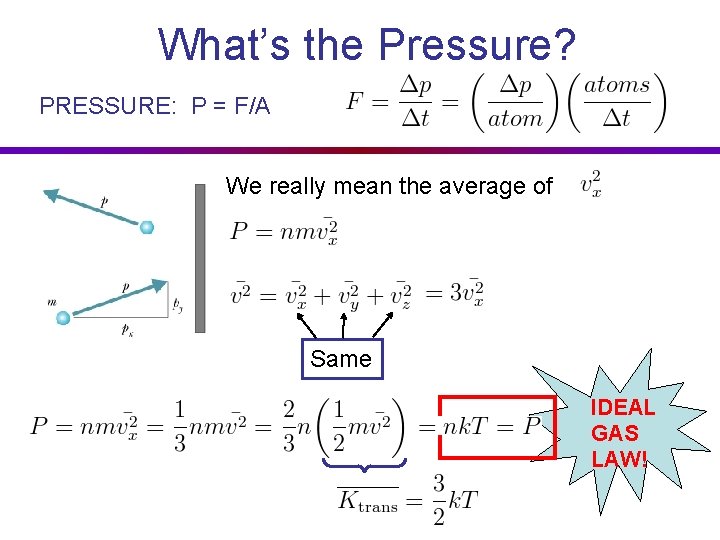
What’s the Pressure? PRESSURE: P = F/A We really mean the average of Same IDEAL GAS LAW!

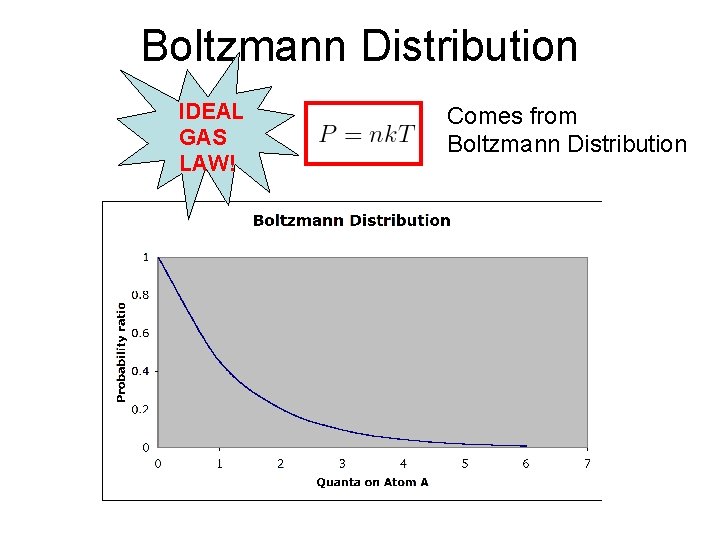
Boltzmann Distribution IDEAL GAS LAW! Comes from Boltzmann Distribution

CLICKER QUESTION A large, standard gas cylinder (tank) has a volume of about 100 liters and is filled with nitrogen gas to a pressure of 2500 psi. Somebody opens the valve and all the nitrogen escapes. Choose the correct statement: A. The tank weighed less after the nitrogen escaped. B. The weight of the tank did not change, because the nitrogen gas molecules don’t rest on the bottom of the tank. C. The weight of the tank did not change, because any nitrogen gas molecules striking the bottom of the tank are balanced by nitrogen gas molecules striking the top of the tank.

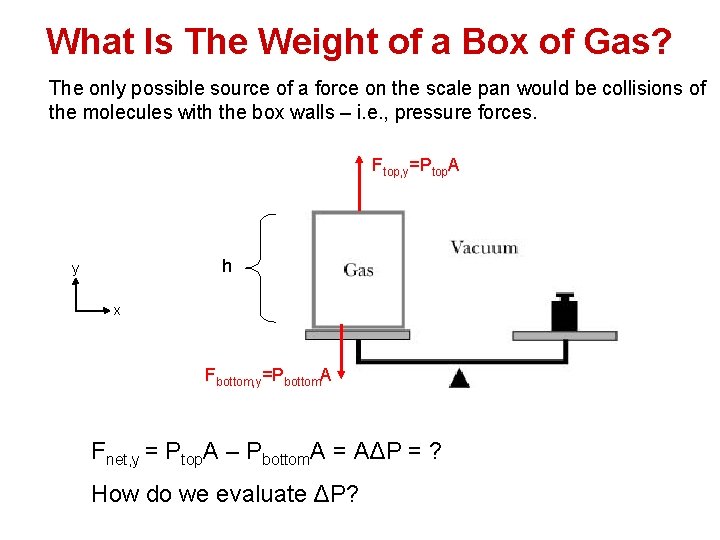
What Is The Weight of a Box of Gas? The only possible source of a force on the scale pan would be collisions of the molecules with the box walls – i. e. , pressure forces. Ftop, y=Ptop. A h y x Fbottom, y=Pbottom. A Fnet, y = Ptop. A – Pbottom. A = AΔP = ? How do we evaluate ΔP?

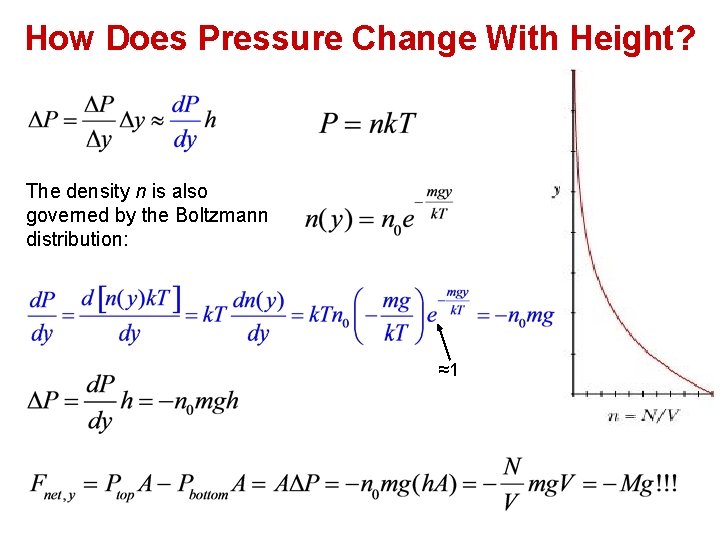
How Does Pressure Change With Height? The density n is also governed by the Boltzmann distribution: ≈1

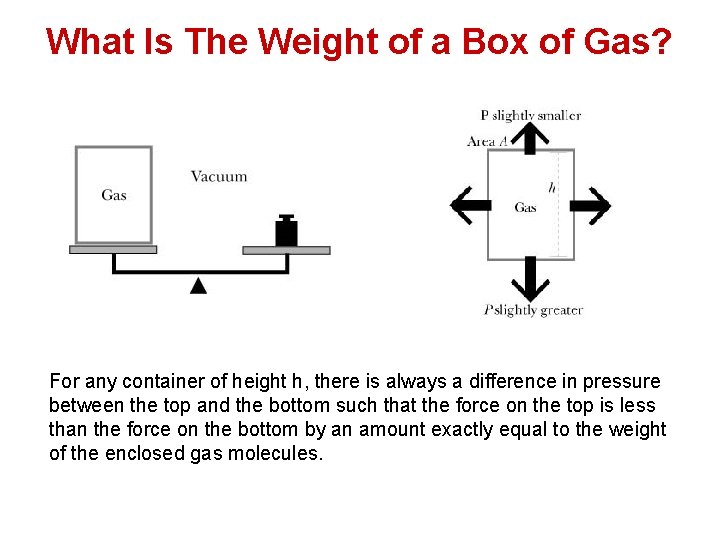
What Is The Weight of a Box of Gas? For any container of height h, there is always a difference in pressure between the top and the bottom such that the force on the top is less than the force on the bottom by an amount exactly equal to the weight of the enclosed gas molecules.

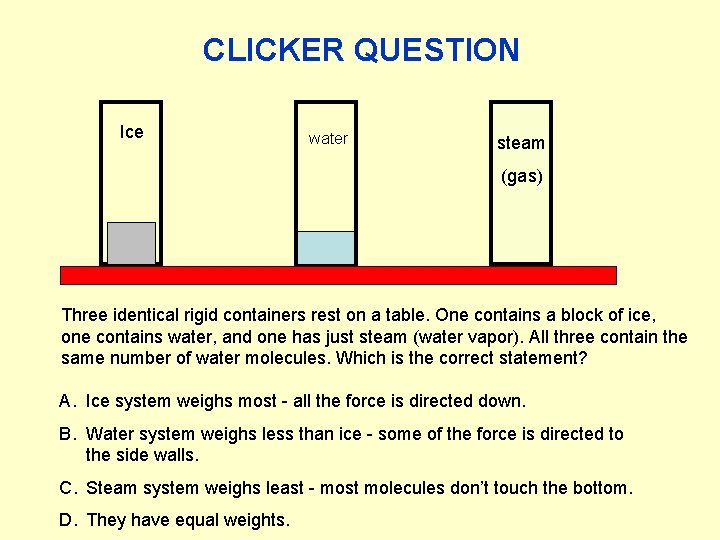
CLICKER QUESTION Ice water steam (gas) Three identical rigid containers rest on a table. One contains a block of ice, one contains water, and one has just steam (water vapor). All three contain the same number of water molecules. Which is the correct statement? A. Ice system weighs most - all the force is directed down. B. Water system weighs less than ice - some of the force is directed to the side walls. C. Steam system weighs least - most molecules don’t touch the bottom. D. They have equal weights.
 Purdue physics 172 past exams
Purdue physics 172 past exams Particl clicker
Particl clicker Iterative prediction of motion
Iterative prediction of motion Phys 172
Phys 172 172+172+283+283
172+172+283+283 Basic electricity and optics
Basic electricity and optics 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Uiuc phys 102
Uiuc phys 102 Phys 101 uiuc
Phys 101 uiuc Ucsd physics courses
Ucsd physics courses How to calculate experimental uncertainty
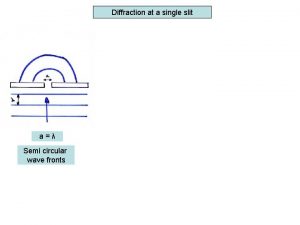
How to calculate experimental uncertainty Single slit envelope
Single slit envelope Http //www.phys.hawaii.edu/ teb/optics/java/slitdiffr/
Http //www.phys.hawaii.edu/ teb/optics/java/slitdiffr/ Phys 212 equation sheet
Phys 212 equation sheet Phys 398 uiuc
Phys 398 uiuc Coloumb units
Coloumb units Att phys medical abbreviation
Att phys medical abbreviation 25 spelling
25 spelling Mastering physics login
Mastering physics login Phys 214
Phys 214