PHY 1039 Properties of Matter Heat Capacity of



- Slides: 16

PHY 1039 Properties of Matter Heat Capacity of Ideal Gases (CP and CV) and Adiabatic Expansion of Ideal Gas (See Finn’s Thermal Physics, Ch. 4) March 12 and 15, 2012 Lectures 11 and 12

From next week: Lectures on Monday at 3 pm will meet in Lecture Theatre E. Week 7 (next week) only: Lecture on Monday, March 19 at 4 pm in 35 AC 04 (instead of tutorial) Tutorial will be held on Thursday, March 22 at 9 am in the Austin Pearce Building, Lab 2 (AP Lab 2)

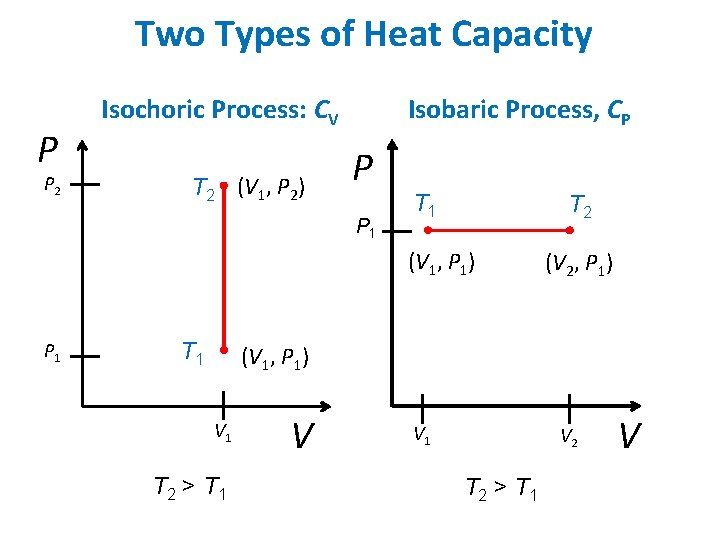
Two Types of Heat Capacity P P 2 Isochoric Process: CV T 2 (V 1, P 2) Isobaric Process, CP P P 1 T 2 (V 1, P 1) P 1 T 1 (V 2, P 1) (V 1, P 1) V 1 T 2 > T 1 V V 1 V 2 T 2 > T 1 V

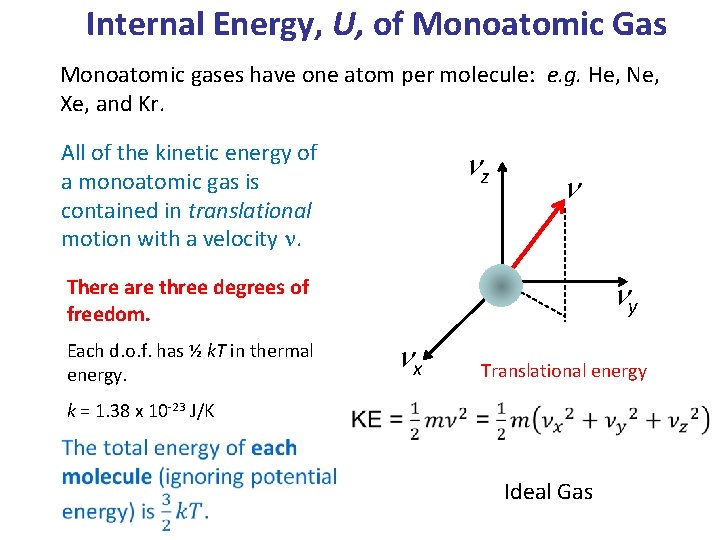
Internal Energy, U, of Monoatomic Gas Monoatomic gases have one atom per molecule: e. g. He, Ne, Xe, and Kr. All of the kinetic energy of a monoatomic gas is contained in translational motion with a velocity n. nz n ny There are three degrees of freedom. nx Each d. o. f. has ½ k. T in thermal energy. k = 1. 38 x 10 -23 J/K Translational energy Ideal Gas

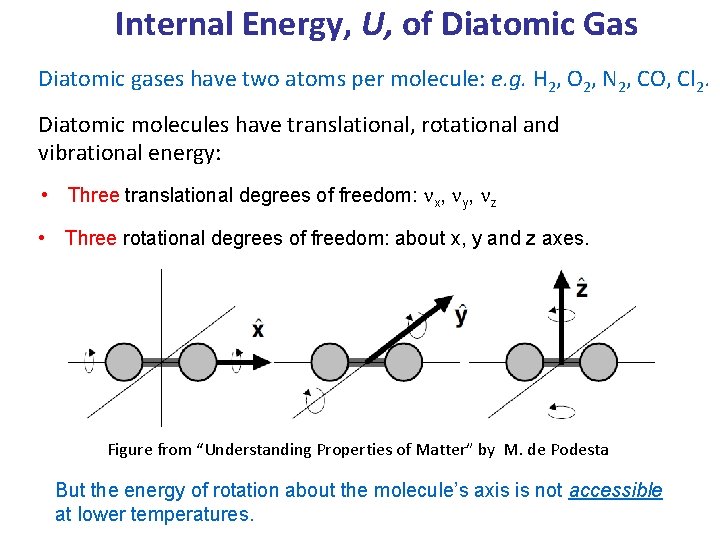
Internal Energy, U, of Diatomic Gas Diatomic gases have two atoms per molecule: e. g. H 2, O 2, N 2, CO, Cl 2. Diatomic molecules have translational, rotational and vibrational energy: • Three translational degrees of freedom: nx, ny, nz • Three rotational degrees of freedom: about x, y and z axes. Figure from “Understanding Properties of Matter” by M. de Podesta But the energy of rotation about the molecule’s axis is not accessible at lower temperatures.

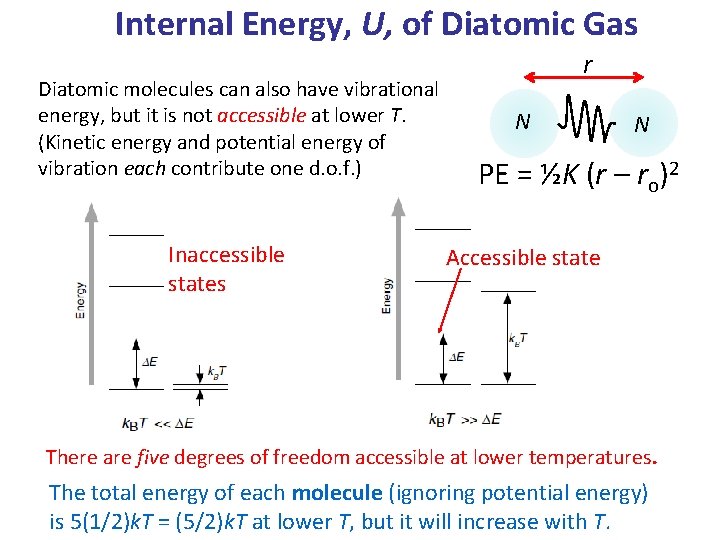
Internal Energy, U, of Diatomic Gas Diatomic molecules can also have vibrational energy, but it is not accessible at lower T. (Kinetic energy and potential energy of vibration each contribute one d. o. f. ) Inaccessible states r N N PE = ½K (r – ro)2 Accessible state There are five degrees of freedom accessible at lower temperatures. The total energy of each molecule (ignoring potential energy) is 5(1/2)k. T = (5/2)k. T at lower T, but it will increase with T.

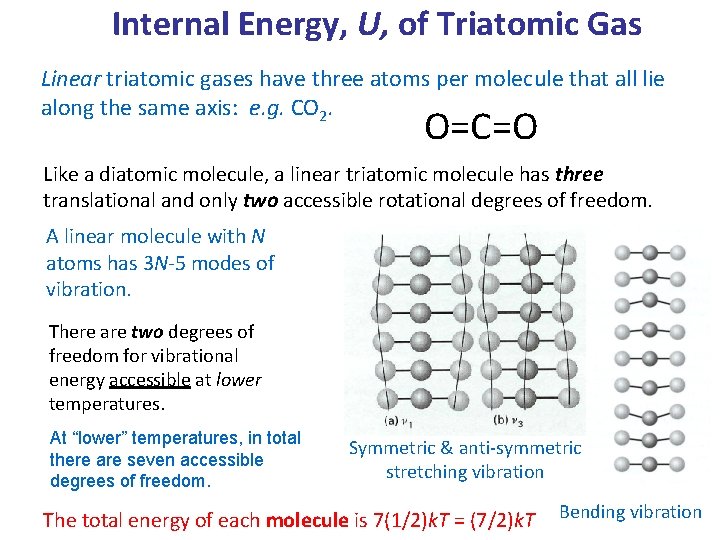
Internal Energy, U, of Triatomic Gas Linear triatomic gases have three atoms per molecule that all lie along the same axis: e. g. CO 2. O=C=O Like a diatomic molecule, a linear triatomic molecule has three translational and only two accessible rotational degrees of freedom. A linear molecule with N atoms has 3 N-5 modes of vibration. There are two degrees of freedom for vibrational energy accessible at lower temperatures. At “lower” temperatures, in total there are seven accessible degrees of freedom. Symmetric & anti-symmetric stretching vibration The total energy of each molecule is 7(1/2)k. T = (7/2)k. T Bending vibration

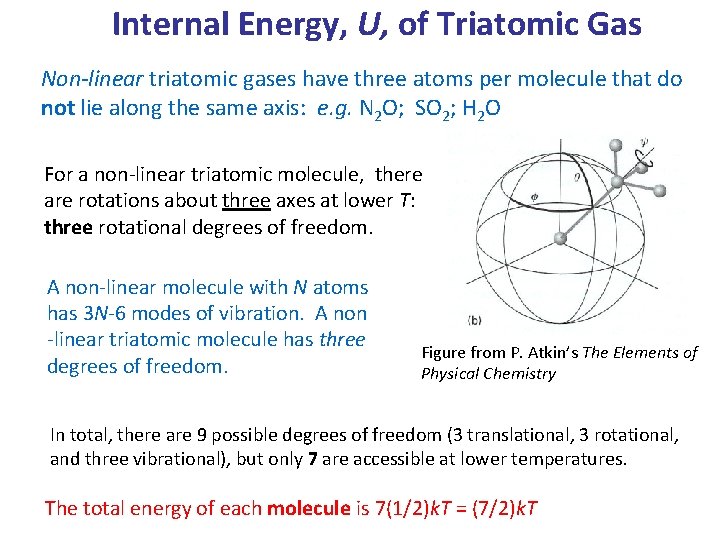
Internal Energy, U, of Triatomic Gas Non-linear triatomic gases have three atoms per molecule that do not lie along the same axis: e. g. N 2 O; SO 2; H 2 O For a non-linear triatomic molecule, there are rotations about three axes at lower T: three rotational degrees of freedom. A non-linear molecule with N atoms has 3 N-6 modes of vibration. A non -linear triatomic molecule has three degrees of freedom. Figure from P. Atkin’s The Elements of Physical Chemistry In total, there are 9 possible degrees of freedom (3 translational, 3 rotational, and three vibrational), but only 7 are accessible at lower temperatures. The total energy of each molecule is 7(1/2)k. T = (7/2)k. T

Greenhouse Effect: A Problem of Thermodynamics http: //www. dailymail. co. uk/sciencetech/article-483191/Arctic-ice-cap-melts-smallest-size. html http: //jcwinnie. biz/wordpress/? p=2235 Earth can be treated as a thermodynamic system.

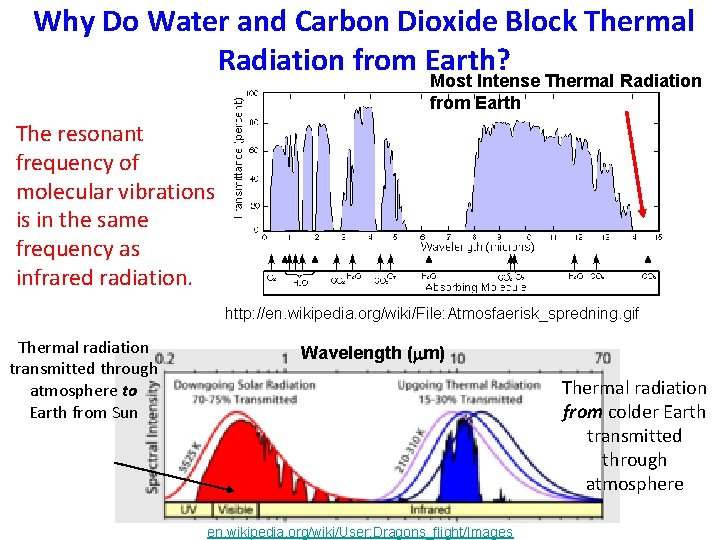
Why Do Water and Carbon Dioxide Block Thermal Radiation from Earth? Most Intense Thermal Radiation from Earth The resonant frequency of molecular vibrations is in the same frequency as infrared radiation. http: //en. wikipedia. org/wiki/File: Atmosfaerisk_spredning. gif Thermal radiation transmitted through atmosphere to Earth from Sun Wavelength (mm) Thermal radiation from colder Earth transmitted through atmosphere en. wikipedia. org/wiki/User: Dragons_flight/Images

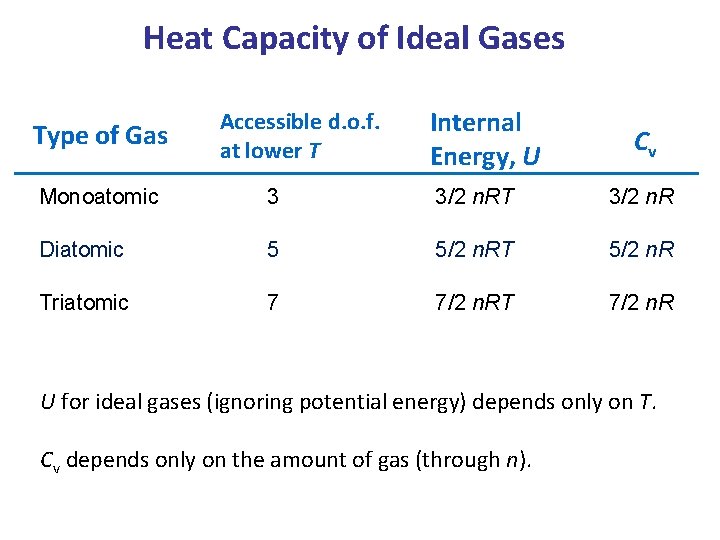
Heat Capacity of Ideal Gases Type of Gas Accessible d. o. f. at lower T Internal Energy, U Cv Monoatomic 3 3/2 n. RT 3/2 n. R Diatomic 5 5/2 n. RT 5/2 n. R Triatomic 7 7/2 n. RT 7/2 n. R U for ideal gases (ignoring potential energy) depends only on T. Cv depends only on the amount of gas (through n).

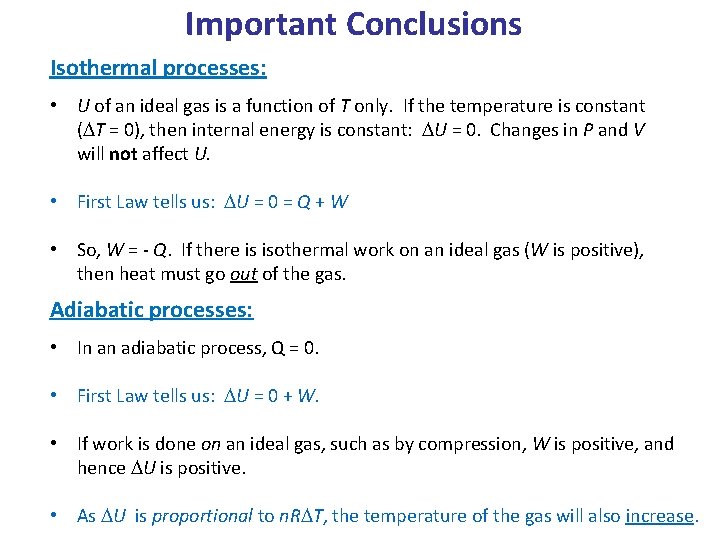
Important Conclusions Isothermal processes: • U of an ideal gas is a function of T only. If the temperature is constant (DT = 0), then internal energy is constant: DU = 0. Changes in P and V will not affect U. • First Law tells us: DU = 0 = Q + W • So, W = - Q. If there is isothermal work on an ideal gas (W is positive), then heat must go out of the gas. Adiabatic processes: • In an adiabatic process, Q = 0. • First Law tells us: DU = 0 + W. • If work is done on an ideal gas, such as by compression, W is positive, and hence DU is positive. • As DU is proportional to n. RDT, the temperature of the gas will also increase.

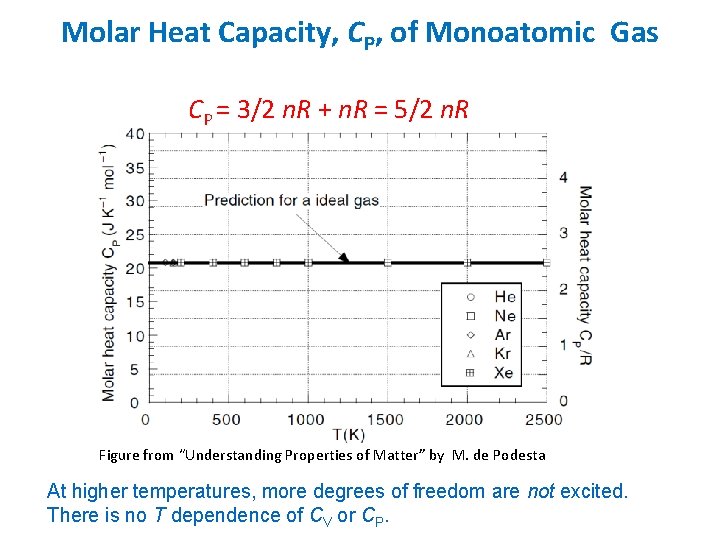
Molar Heat Capacity, CP, of Monoatomic Gas CP = 3/2 n. R + n. R = 5/2 n. R Figure from “Understanding Properties of Matter” by M. de Podesta At higher temperatures, more degrees of freedom are not excited. There is no T dependence of CV or CP.

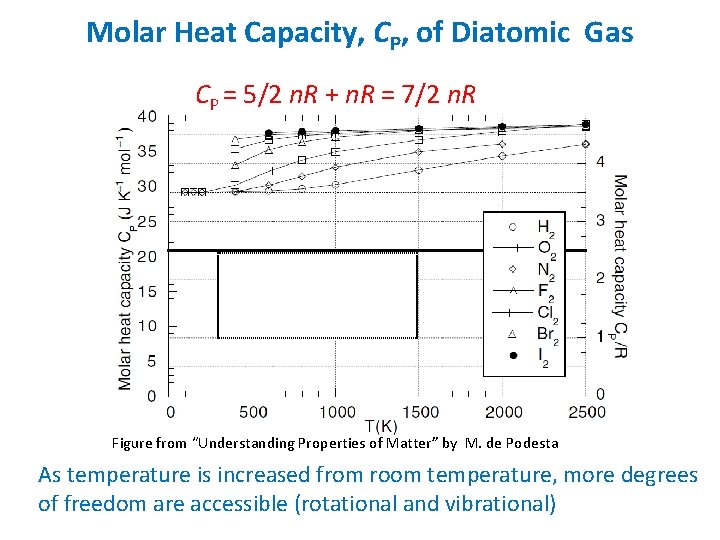
Molar Heat Capacity, CP, of Diatomic Gas CP = 5/2 n. R + n. R = 7/2 n. R Figure from “Understanding Properties of Matter” by M. de Podesta As temperature is increased from room temperature, more degrees of freedom are accessible (rotational and vibrational)

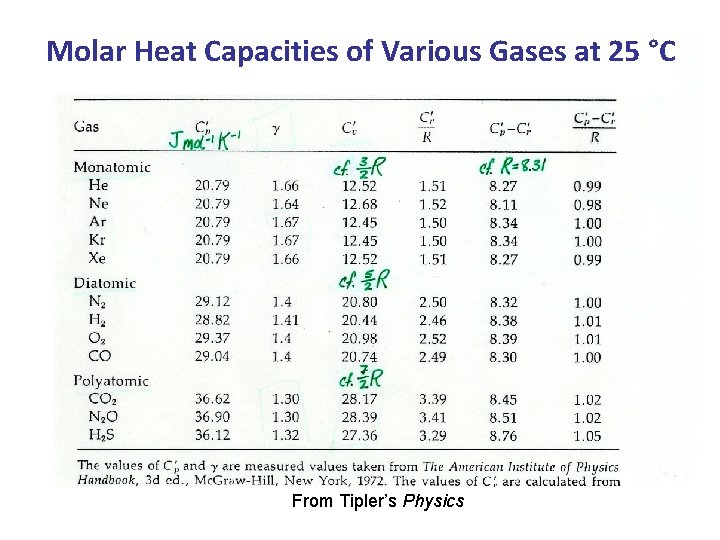
Molar Heat Capacities of Various Gases at 25 °C From Tipler’s Physics

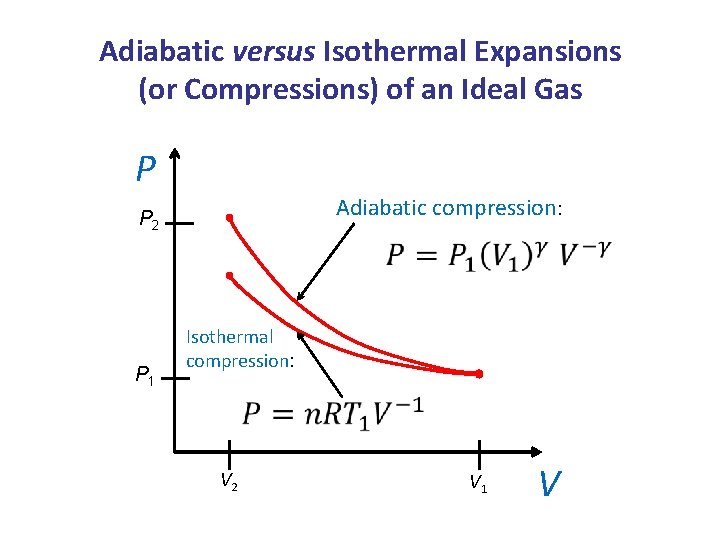
Adiabatic versus Isothermal Expansions (or Compressions) of an Ideal Gas P Adiabatic compression: P 2 P 1 Isothermal compression: V 2 V 1 V