Phenolderived Products from Fast Pyrolysis of Organosolv Lignin


- Slides: 15

Phenol-derived Products from Fast Pyrolysis of Organosolv Lignin Kanit Soongprasit, Ph. D Assoc. Prof. Viboon Sricharoenchaikul, Ph. D Duangduen Atong, Ph. D National Metal and Materials Technology Center (MTEC) Faculty of Engineering, Department of Environmental Engineering, Chulalongkorn University

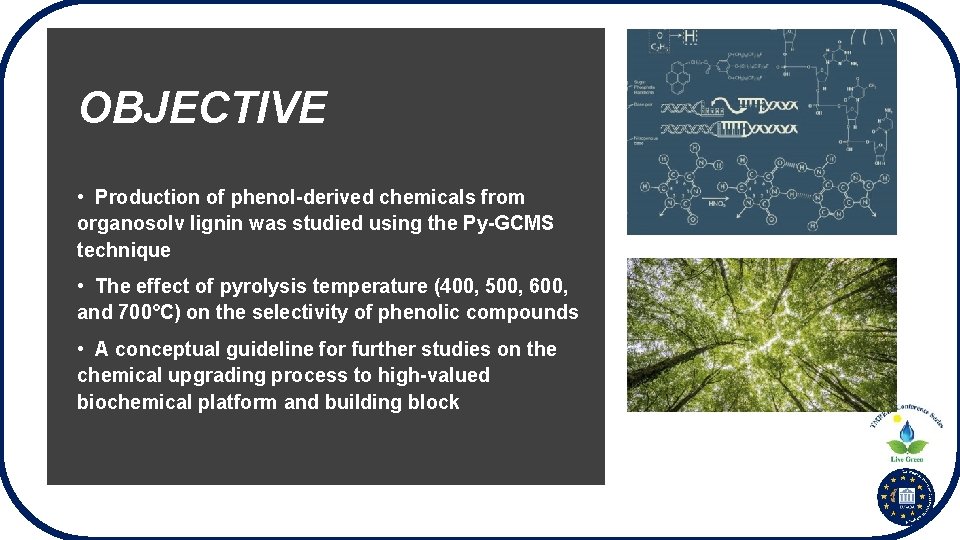
OBJECTIVE • Production of phenol-derived chemicals from organosolv lignin was studied using the Py-GCMS technique • The effect of pyrolysis temperature (400, 500, 600, and 700°C) on the selectivity of phenolic compounds • A conceptual guideline for further studies on the chemical upgrading process to high-valued biochemical platform and building block

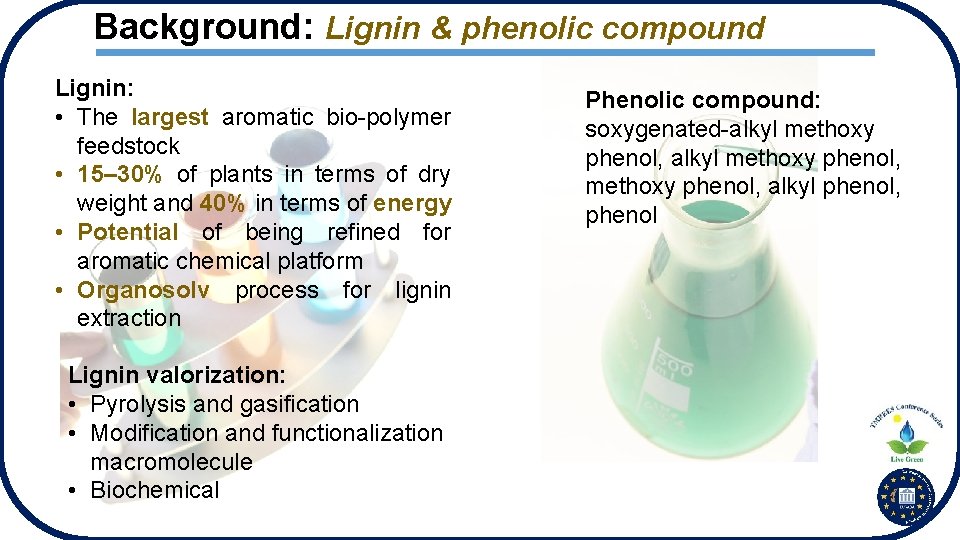
Background: Lignin & phenolic compound Lignin: • The largest aromatic bio-polymer feedstock • 15– 30% of plants in terms of dry weight and 40% in terms of energy • Potential of being refined for aromatic chemical platform • Organosolv process for lignin extraction Lignin valorization: • Pyrolysis and gasification • Modification and functionalization macromolecule • Biochemical Phenolic compound: soxygenated-alkyl methoxy phenol, alkyl phenol, phenol

Experimental: Sample preparation Sugarcane bagasse (BG) Dried Pulverized Sieved (125– 425µm) Organosolv lignin extraction process 1. Autogenous conditions with Et. OH and H 2 O in the ratio 75: 25 2. 10 g of BG was physically mixed and soaked with the extracting agent 3. The mixing precursor was poured into a 50 ml PTFE- stainless steel autoclave 4. Hot air oven at a temperature of 180ºC for 3 hours 5. Filtered using a filter paper with 5– 13 µm pore diameter 6. Dried at 60ºC Analyzer technique: • Thermogravimetric analyzer (METTLER TOLEDO; TGA 2). • Fourier-transform infrared spectroscopy (Perkin Elmer; Spectrum Spotlight 300; FTIR) • Nuclear magnetic resonance spectroscopy (BRUKER; AV 500; NMR).

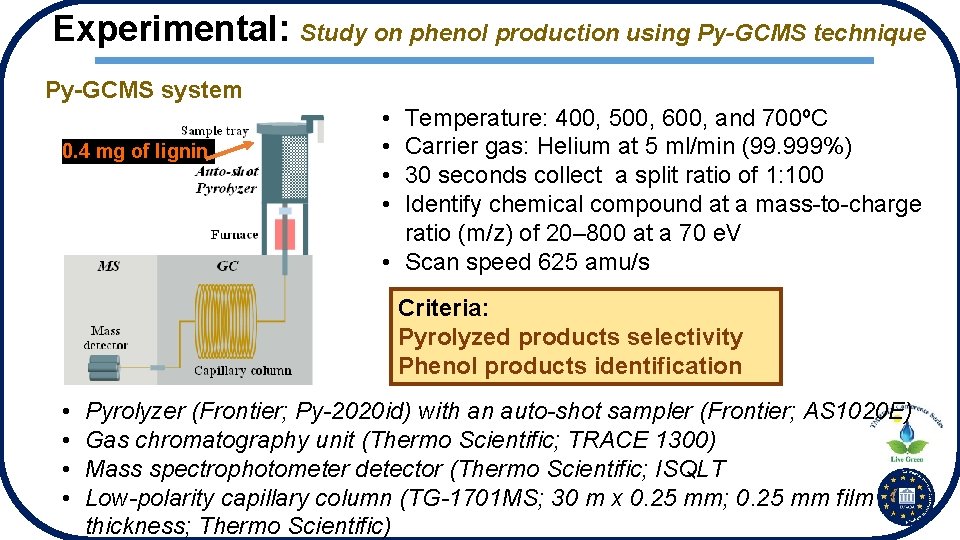
Experimental: Study on phenol production using Py-GCMS technique Py-GCMS system 0. 4 mg of lignin • • Temperature: 400, 500, 600, and 700ºC Carrier gas: Helium at 5 ml/min (99. 999%) 30 seconds collect a split ratio of 1: 100 Identify chemical compound at a mass-to-charge ratio (m/z) of 20– 800 at a 70 e. V • Scan speed 625 amu/s Criteria: Pyrolyzed products selectivity Phenol products identification • • Pyrolyzer (Frontier; Py-2020 id) with an auto-shot sampler (Frontier; AS 1020 E) Gas chromatography unit (Thermo Scientific; TRACE 1300) Mass spectrophotometer detector (Thermo Scientific; ISQLT Low-polarity capillary column (TG-1701 MS; 30 m x 0. 25 mm; 0. 25 mm film thickness; Thermo Scientific)

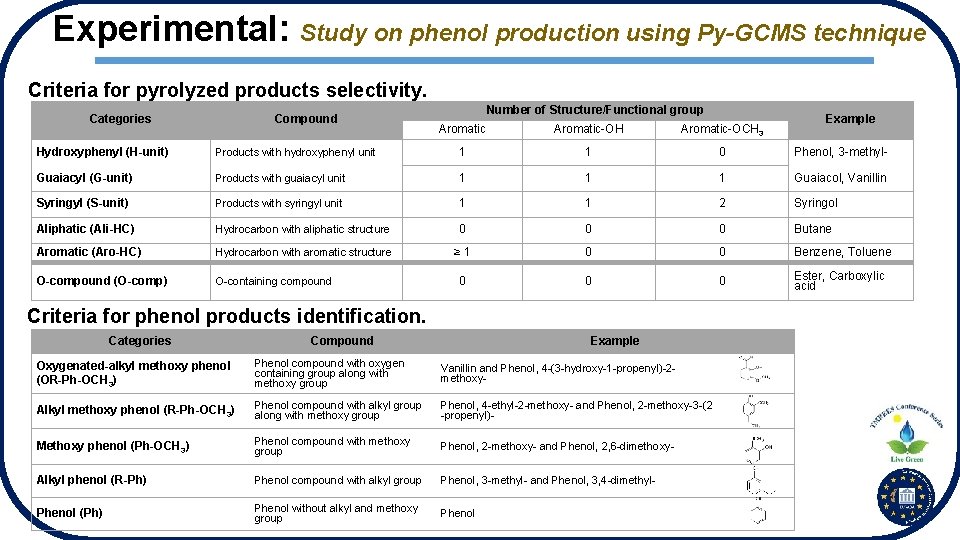
Experimental: Study on phenol production using Py-GCMS technique Criteria for pyrolyzed products selectivity. Categories Compound Number of Structure/Functional group Aromatic-OH Aromatic-OCH 3 Example Hydroxyphenyl (H-unit) Products with hydroxyphenyl unit 1 1 0 Phenol, 3 -methyl- Guaiacyl (G-unit) Products with guaiacyl unit 1 1 1 Guaiacol, Vanillin Syringyl (S-unit) Products with syringyl unit 1 1 2 Syringol Aliphatic (Ali-HC) Hydrocarbon with aliphatic structure 0 0 0 Butane Aromatic (Aro-HC) Hydrocarbon with aromatic structure ≥ 1 0 0 Benzene, Toluene O-compound (O-comp) O-containing compound 0 0 0 Ester, Carboxylic acid Criteria for phenol products identification. Categories Compound Example Oxygenated-alkyl methoxy phenol (OR-Ph-OCH 3) Phenol compound with oxygen containing group along with methoxy group Vanillin and Phenol, 4 -(3 -hydroxy-1 -propenyl)-2 methoxy- Alkyl methoxy phenol (R-Ph-OCH 3) Phenol compound with alkyl group along with methoxy group Phenol, 4 -ethyl-2 -methoxy- and Phenol, 2 -methoxy-3 -(2 -propenyl)- Methoxy phenol (Ph-OCH 3) Phenol compound with methoxy group Phenol, 2 -methoxy- and Phenol, 2, 6 -dimethoxy- Alkyl phenol (R-Ph) Phenol compound with alkyl group Phenol, 3 -methyl- and Phenol, 3, 4 -dimethyl- Phenol (Ph) Phenol without alkyl and methoxy group Phenol

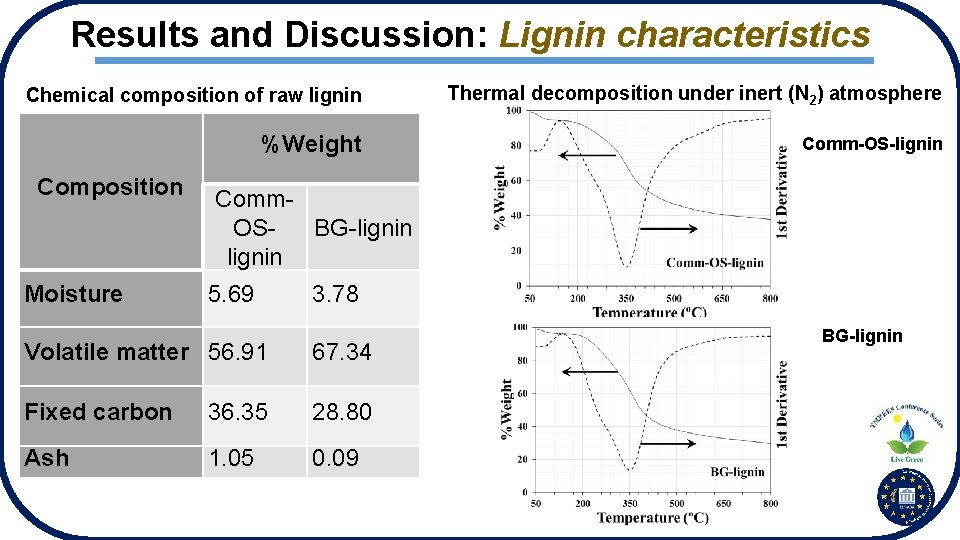
Results and Discussion: Lignin characteristics Chemical composition of raw lignin Thermal decomposition under inert (N 2) atmosphere %Weight Comm-OS-lignin Composition Moisture Comm. OSBG-lignin 5. 69 3. 78 Volatile matter 56. 91 67. 34 Fixed carbon 36. 35 28. 80 Ash 1. 05 0. 09 BG-lignin

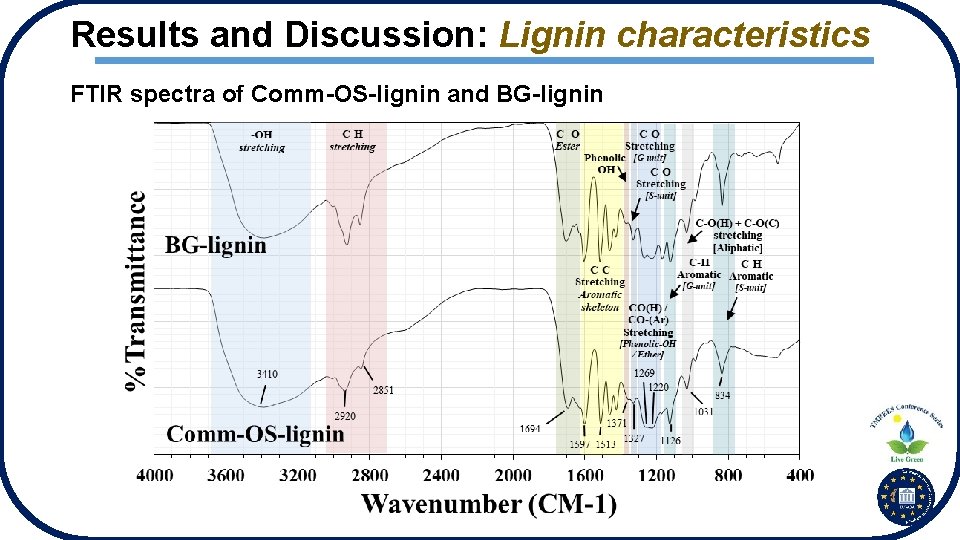
Results and Discussion: Lignin characteristics FTIR spectra of Comm-OS-lignin and BG-lignin

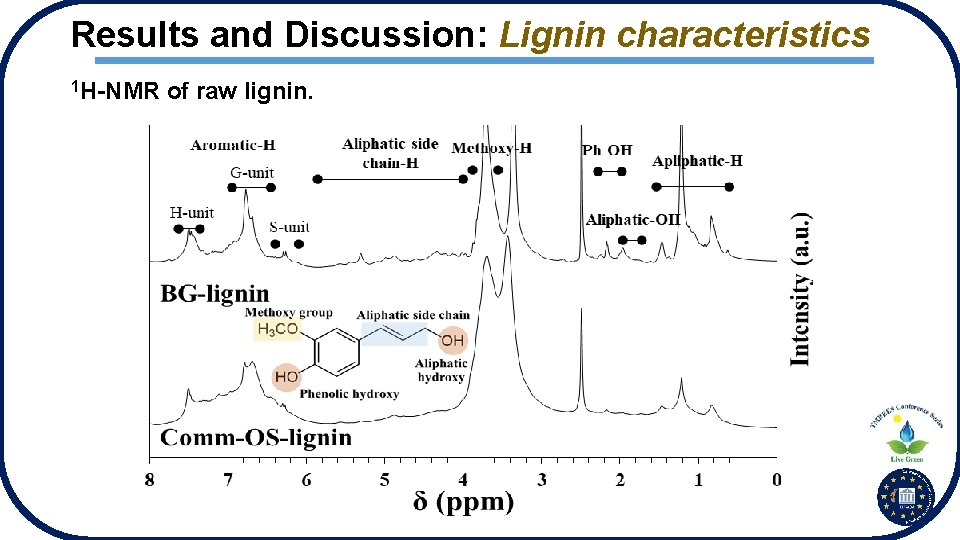
Results and Discussion: Lignin characteristics 1 H-NMR of raw lignin.

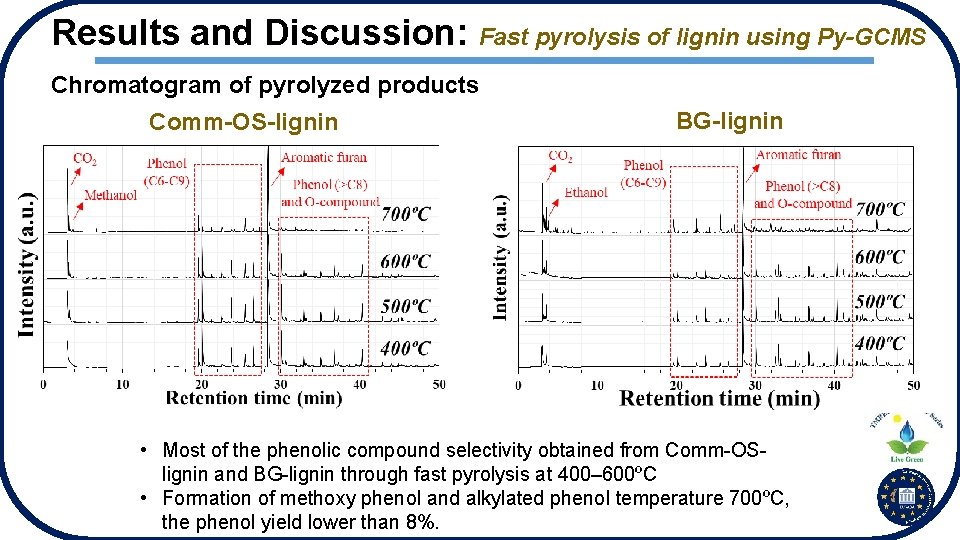
Results and Discussion: Fast pyrolysis of lignin using Py-GCMS Chromatogram of pyrolyzed products Comm-OS-lignin BG-lignin • Most of the phenolic compound selectivity obtained from Comm-OSlignin and BG-lignin through fast pyrolysis at 400– 600ºC • Formation of methoxy phenol and alkylated phenol temperature 700ºC, the phenol yield lower than 8%.

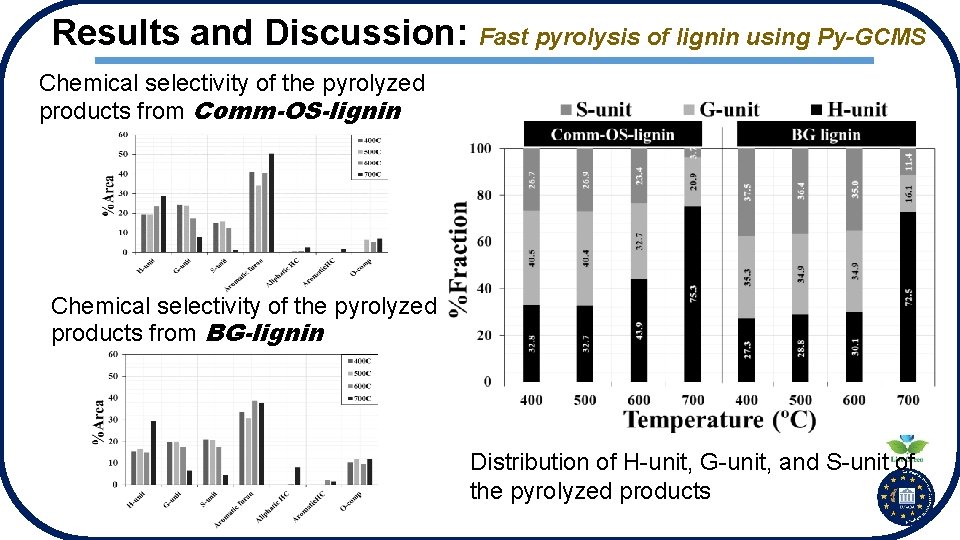
Results and Discussion: Fast pyrolysis of lignin using Py-GCMS Chemical selectivity of the pyrolyzed products from Comm-OS-lignin Chemical selectivity of the pyrolyzed products from BG-lignin Distribution of H-unit, G-unit, and S-unit of the pyrolyzed products

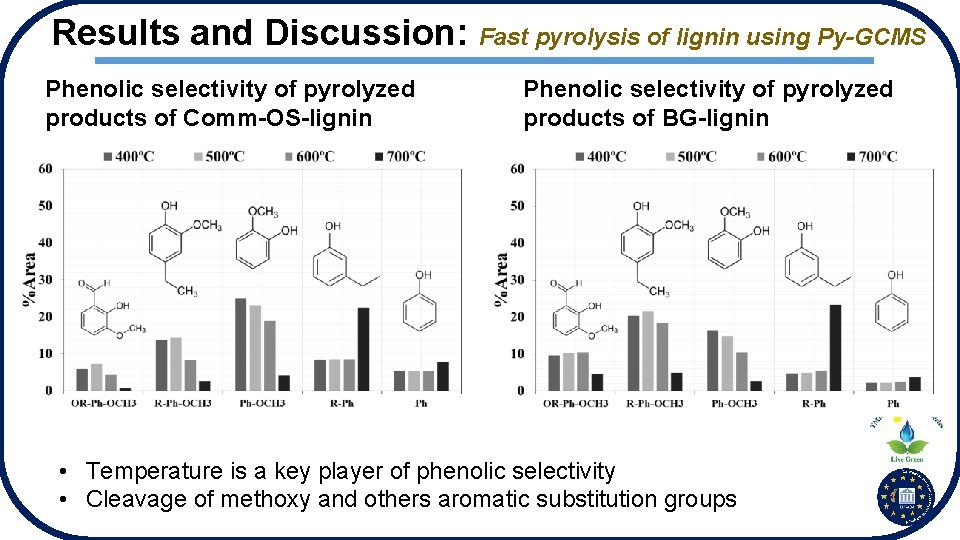
Results and Discussion: Fast pyrolysis of lignin using Py-GCMS Phenolic selectivity of pyrolyzed products of Comm-OS-lignin Phenolic selectivity of pyrolyzed products of BG-lignin • Temperature is a key player of phenolic selectivity • Cleavage of methoxy and others aromatic substitution groups

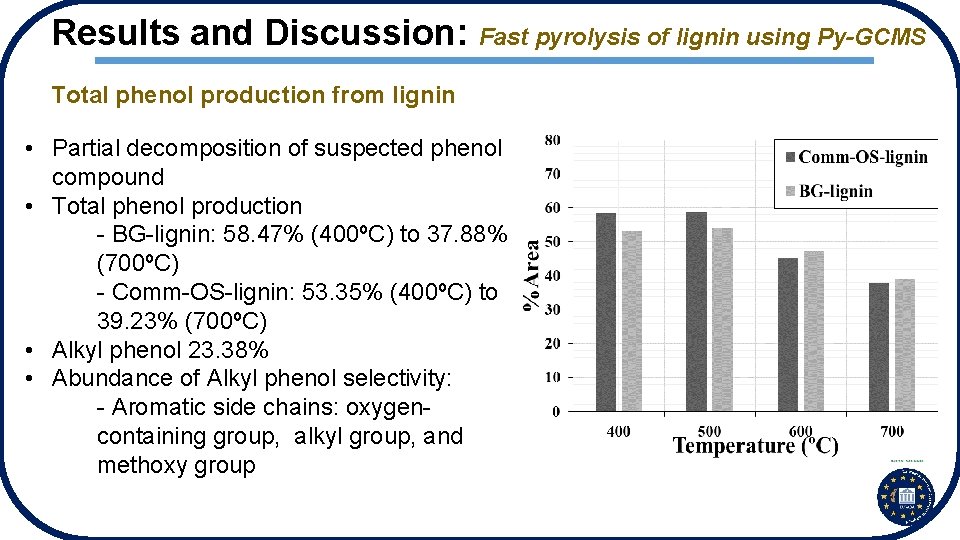
Results and Discussion: Fast pyrolysis of lignin using Py-GCMS Total phenol production from lignin • Partial decomposition of suspected phenol compound • Total phenol production - BG-lignin: 58. 47% (400ºC) to 37. 88% (700ºC) - Comm-OS-lignin: 53. 35% (400ºC) to 39. 23% (700ºC) • Alkyl phenol 23. 38% • Abundance of Alkyl phenol selectivity: - Aromatic side chains: oxygencontaining group, alkyl group, and methoxy group

Conclusion • Bagasse from the local industry as raw materials (BG-lignin) evaluated with commercial organosolv lignin (Comm-OS-lignin) • BG-lignin exhibited in term of volatile organic compounds (VOCs) and fixed carbon of 93. 26% and 96. 14% • BG-linin significantly lower ash of 0. 09% • Highest degree of Comm-OS-lignin and BG-lignin degradation at a temperature of about 350ºC • Demethoxylation reaction of methoxy group of G-unit and S-unit, high temperature that results in a vastly enhanced H-unit and slightly on aromatic hydrocarbon products • A higher pyrolysis temperature of 700ºC, the elimination of aromatic side group and promoted alkyl phenol selectivity • Selective catalysts, such as vanillin, BTX, and other aromatics, upgrade the valued chemical production for chemistry industrial, food, pharmaceutical, and cosmetic industries.

 Oorja systems & consultants
Oorja systems & consultants Types of fibers forensics
Types of fibers forensics Structure of lignin
Structure of lignin A simple mechanical tissue devoid of lignin is
A simple mechanical tissue devoid of lignin is Lignin vzorec
Lignin vzorec Purehemp technology
Purehemp technology Zat lignin
Zat lignin Acid fast vs non acid fast
Acid fast vs non acid fast Example of acid-fast bacteria
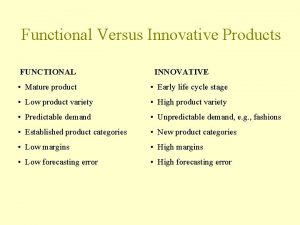
Example of acid-fast bacteria Functional and innovative products supply chain
Functional and innovative products supply chain Coke vs pepsi sales
Coke vs pepsi sales Rodzaje fast foodów
Rodzaje fast foodów Fastgalp com
Fastgalp com Ncfast log in
Ncfast log in Straight dough method
Straight dough method Fast avc
Fast avc