EVALUATION OF SOFTWOOD KRAFT AND ORGANOSOLV LIGNIN AS



- Slides: 10

EVALUATION OF SOFTWOOD KRAFT AND ORGANOSOLV LIGNIN AS FEEDSTOCK FOR BIOFUELS AND BIOMATERIALS Oihana Gordobil Donostia, 26 th May 2015

Contents • Objectives • Methodology • Results Chemical composition and molecular weight Functional groups (31 P NMR /13 C NMR) Thermal properties Proximate analysis • Conclusions

Objectives Chemical, structural and thermal characterization of organosolv and Kraft Spruce lignins to compare their potential use as feedstock in different applications like bioenergy or biomaterials.

Methodology Isolation of lignins ORGANOSOLV KRAFT Ethanol-water (50%wt) H =1: 7 (w/w) T = 180°C t = 60 min H 2 SO 4 (1. 2 %wt) Lignoboost process SPRUCE Characterization of lignins • Chemical composition (Klason and soluble lignin, ashes, sugar and sulfur content) • SEC (Molecular weight ) • 31 P NMR (Aliphatic and phenolic hydroxyls content) • 13 C NMR (Degree of condensation) • DSC (Glass transition temperature Tg) • TGA (Thermal stability, % of volatiles, Fixed Carbon, Organic matter and Heat capacity) Chemical Structure Thermal properties

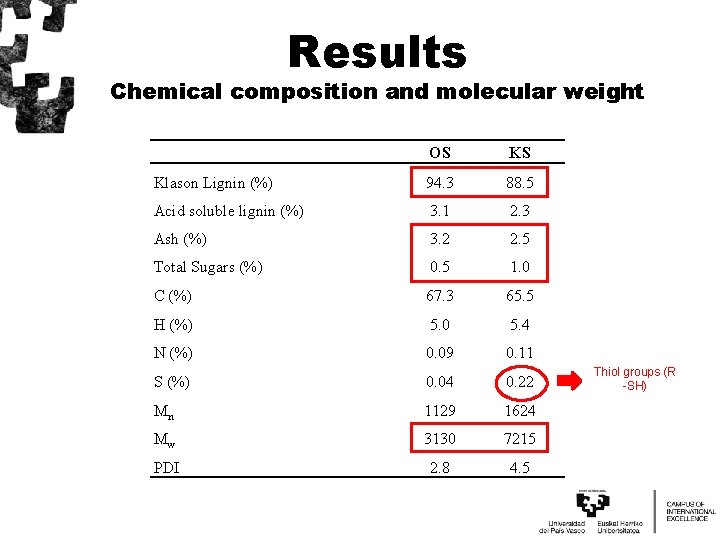
Results Chemical composition and molecular weight OS KS Klason Lignin (%) 94. 3 88. 5 Acid soluble lignin (%) 3. 1 2. 3 Ash (%) 3. 2 2. 5 Total Sugars (%) 0. 5 1. 0 C (%) 67. 3 65. 5 H (%) 5. 0 5. 4 N (%) 0. 09 0. 11 S (%) 0. 04 0. 22 Mn 1129 1624 Mw 3130 7215 PDI 2. 8 4. 5 Thiol groups (R -SH)

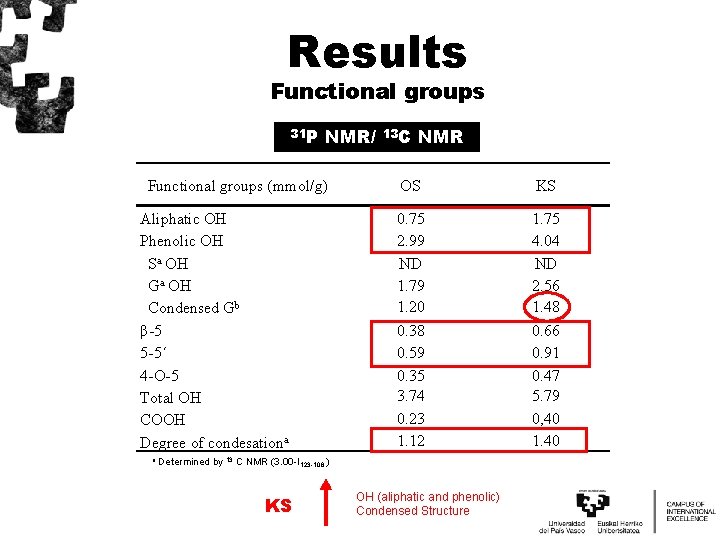
Results Functional groups 31 P NMR/ Functional groups (mmol/g) Aliphatic OH Phenolic OH Sa OH Ga OH Condensed Gb β-5 5 -5´ 4 -O-5 Total OH COOH Degree of condesationa a 13 C NMR OS KS 0. 75 2. 99 ND 1. 79 1. 20 0. 38 0. 59 0. 35 3. 74 0. 23 1. 12 1. 75 4. 04 ND 2. 56 1. 48 0. 66 0. 91 0. 47 5. 79 0, 40 1. 40 Determined by 13 C NMR (3. 00 -I 123 -106 ) KS OH (aliphatic and phenolic) Condensed Structure

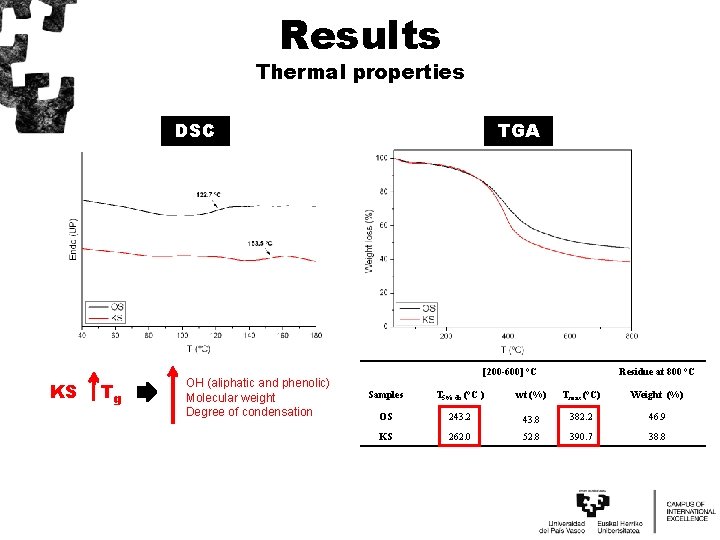
Results Thermal properties DSC KS Tg OH (aliphatic and phenolic) Molecular weight Degree of condensation TGA [200 -600] °C Residue at 800 °C Samples T 5% db (°C ) wt (%) Tmax (°C) Weight (%) OS 243. 2 43. 8 382. 2 46. 9 KS 262. 0 52. 8 390. 7 38. 8

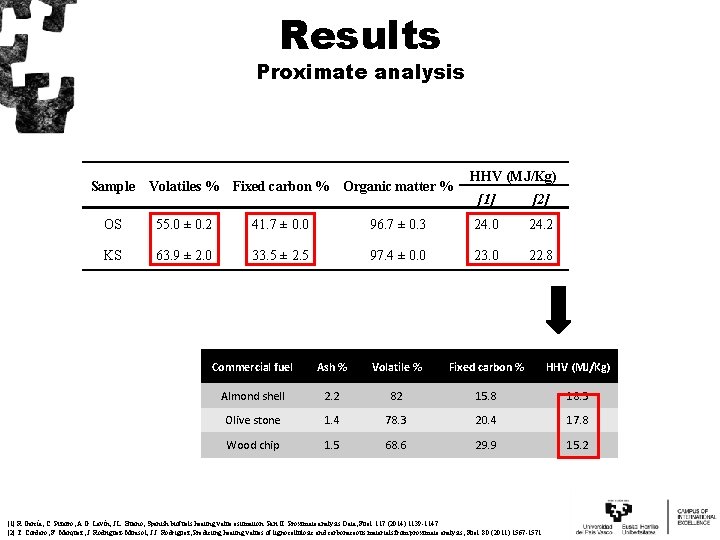
Results Proximate analysis Sample Volatiles % Fixed carbon % Organic matter % HHV (MJ/Kg) [1] [2] OS 55. 0 ± 0. 2 41. 7 ± 0. 0 96. 7 ± 0. 3 24. 0 24. 2 KS 63. 9 ± 2. 0 33. 5 ± 2. 5 97. 4 ± 0. 0 23. 0 22. 8 Commercial fuel Ash % Volatile % Fixed carbon % HHV (MJ/Kg) Almond shell 2. 2 82 15. 8 18. 3 Olive stone 1. 4 78. 3 20. 4 17. 8 Wood chip 1. 5 68. 6 29. 9 15. 2 [1] R. García, C. Pizarro, A. G. Lavín, J. L. Bueno, Spanish biofuels heating value estimation. Part II: Proximate analysis Data, Fuel. 117 (2014) 1139 -1147. [2] T. Cordero, F. Marquez, J. Rodriguez-Mirasol, J. J. Rodriguez, Predicting heating values of lignocellulosic and carbonaceous materials from proximate analysis, Fuel. 80 (2011) 1567 -1571.

Conclusions • Both isolated lignins presented different structure and thermal properties because of the used extraction process. • Both lignins showed high purity. Kraft Lignin (KS) presented the highest sulfur content. • KS had higher Mw than OS and higher molecular weight resulted in higher Tg and thermal stability. • KS had more condensed structure (β-5, 5 -5´ ) than OS which is generated during the last stage of Kraft process and higher content of aliphatic and phenolic hydroxyl groups due to the cleavage of α-aryl and β-aryl ether linkages in native lignin during the Kraft process. • The total content of OH groups was generally high for both lignins, making them a suitable raw material for chemical modifications. The content of phenolic OH groups was higher for Kraft lignin. • Both lignins can be suitable to use in bioenergy applications because they showed appropriate values in the proximate analysis.

Thank you for your attention!