Pharmacokinetics of Extravascular Drug Administration Level Intermediate Version








- Slides: 27

Pharmacokinetics of Extravascular Drug Administration Level : Intermediate Version No: 1 Version Date: October 2013 Prepared by: Balram Chowbay, Ph. D Principal Pharmacologist Clinical Pharmacology Lab National Cancer Center Narrated by: Natalia Sutiman

Disclaimer/Liability • The information provided in the VAP is made available in good faith and is derived from sources believed to be reliable and accurate at the time of release. • The materials presented on the VAP may include links to external Internet sites. These external information sources are outside the control of Duke-NUS. The user of the Internet links is responsible for making his or her own decision about the accuracy, reliability and correctness of the information found. • In no event shall Duke-NUS be liable for any indirect, special, incidental, or consequential damages arising out of any use of reliance of any information contained in the VAP. Nor does Duke-NUS assume any responsibility for failure or delay in updating or removing the information contained in the VAP. • Moreover, information provided on the VAP does not constitute medical advice or treatment nor should it be considered as a replacement of the patient/physician relationship or a physician’s professional judgment. Duke. NUS expressly disclaims all liability for treatment, diagnosis, decisions and actions taken or not taken in reliance upon information contained in the VAP. This work is licensed under a Creative Commons Attribution-Non. Commercial-No. Derivs 3. 0 Unported License To view a copy of this license, visit [http: //creativecommons. org/licenses/by-nc-nd/3. 0/]

Financial Disclosures (past 3 years) • No Disclosures

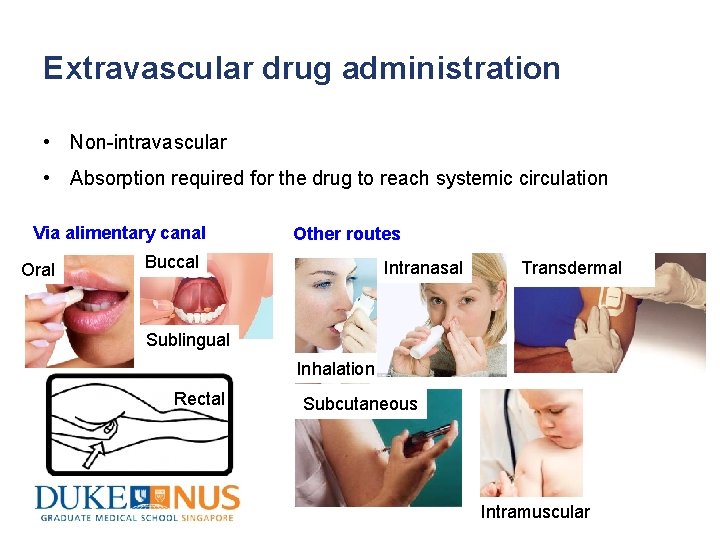
Extravascular drug administration • Non-intravascular • Absorption required for the drug to reach systemic circulation Via alimentary canal Oral Other routes Buccal Intranasal Transdermal Sublingual Inhalation Rectal Subcutaneous Intramuscular

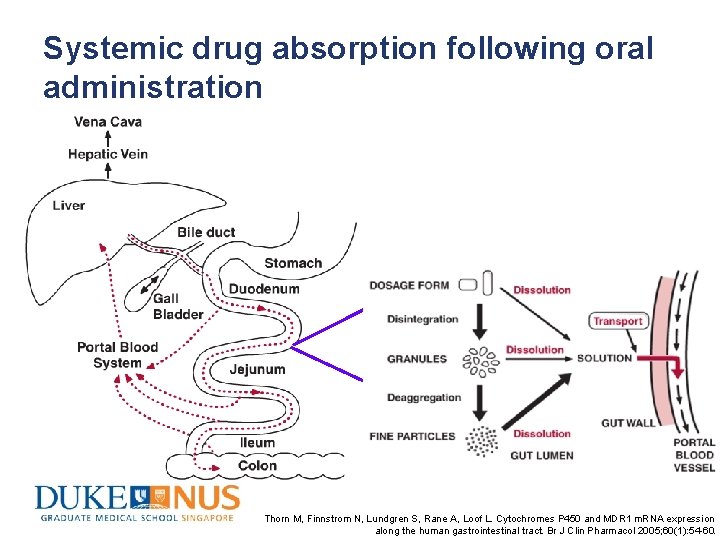
Systemic drug absorption following oral administration Thorn M, Finnstrom N, Lundgren S, Rane A, Loof L. Cytochromes P 450 and MDR 1 m. RNA expression along the human gastrointestinal tract. Br J Clin Pharmacol 2005; 60(1): 54 -60.

Factors influencing release & absorption kinetics

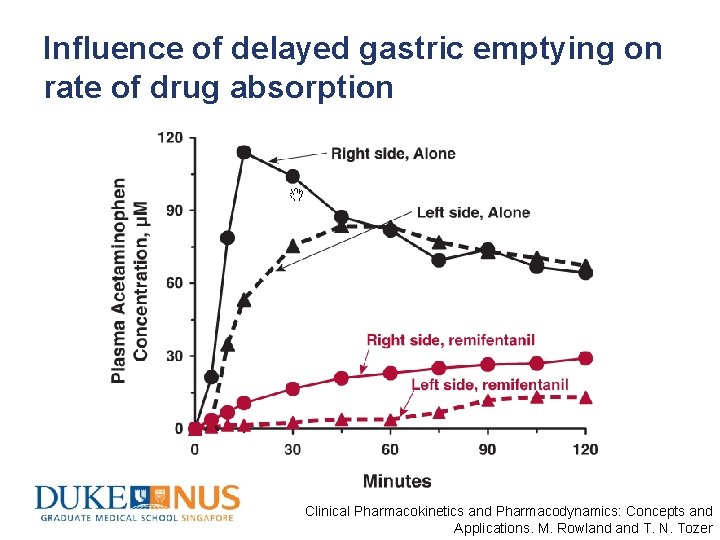
Influence of delayed gastric emptying on rate of drug absorption Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. M. Rowland T. N. Tozer

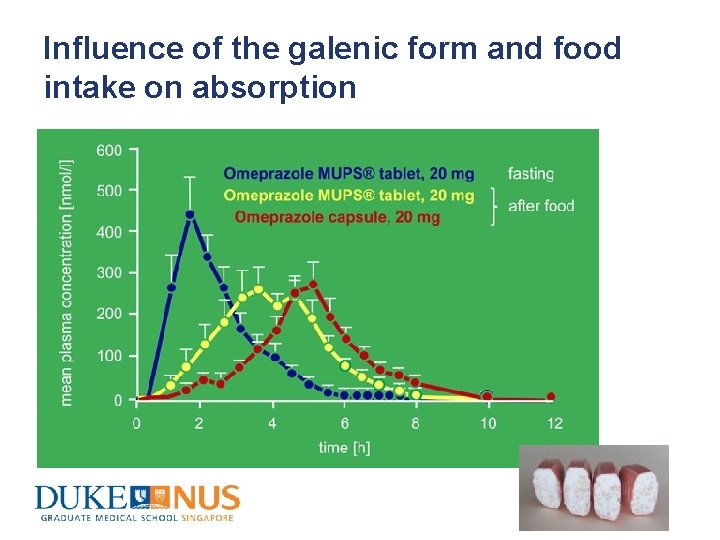
Influence of the galenic form and food intake on absorption

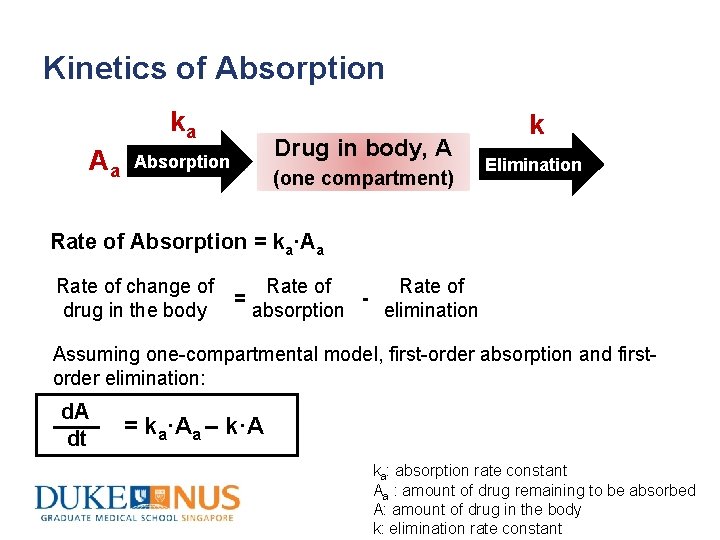
Kinetics of Absorption ka Drug in body, A Absorption Aa Drug (one compartment) k Elimination Rate of Absorption = ka·Aa Rate of change of drug in the body = Rate of elimination absorption Assuming one-compartmental model, first-order absorption and firstorder elimination: d. A dt = ka·Aa – k·A ka: absorption rate constant Aa : amount of drug remaining to be absorbed A: amount of drug in the body k: elimination rate constant

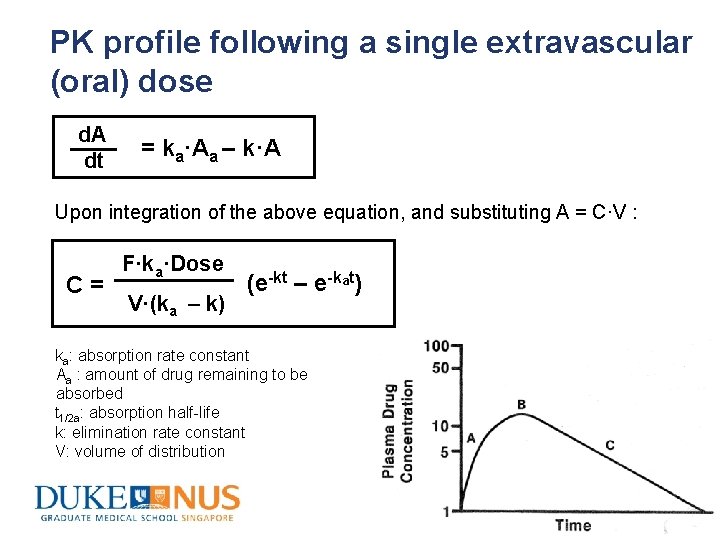
PK profile following a single extravascular (oral) dose d. A dt = ka·Aa – k·A Upon integration of the above equation, and substituting A = C·V : C= F·ka·Dose V·(ka – k) (e-kt – e-kat) ka: absorption rate constant Aa : amount of drug remaining to be absorbed t 1/2 a: absorption half-life k: elimination rate constant V: volume of distribution

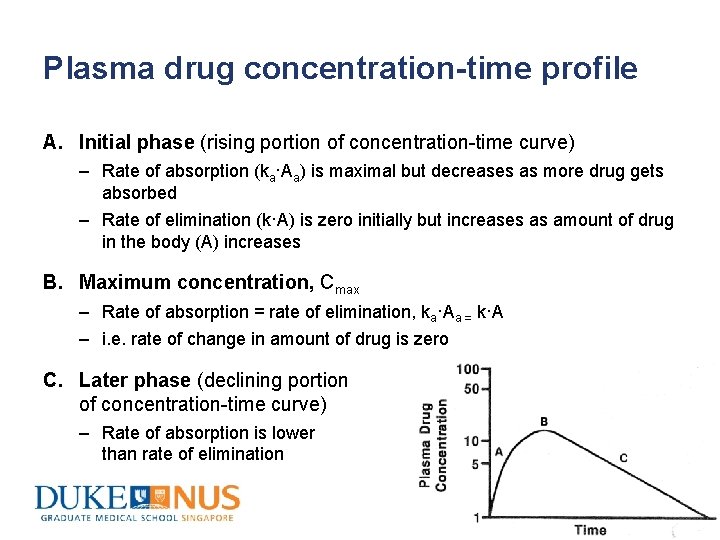
Plasma drug concentration-time profile A. Initial phase (rising portion of concentration-time curve) – Rate of absorption (ka·Aa) is maximal but decreases as more drug gets absorbed – Rate of elimination (k·A) is zero initially but increases as amount of drug in the body (A) increases B. Maximum concentration, Cmax – Rate of absorption = rate of elimination, ka·Aa = k·A – i. e. rate of change in amount of drug is zero C. Later phase (declining portion of concentration-time curve) – Rate of absorption is lower than rate of elimination

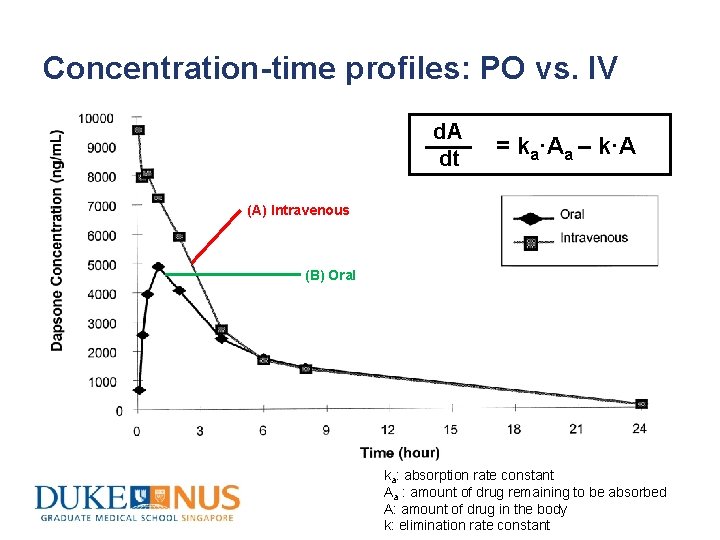
Concentration-time profiles: PO vs. IV d. A dt = ka·Aa – k·A (A) Intravenous (B) Oral ka: absorption rate constant Aa : amount of drug remaining to be absorbed A: amount of drug in the body k: elimination rate constant

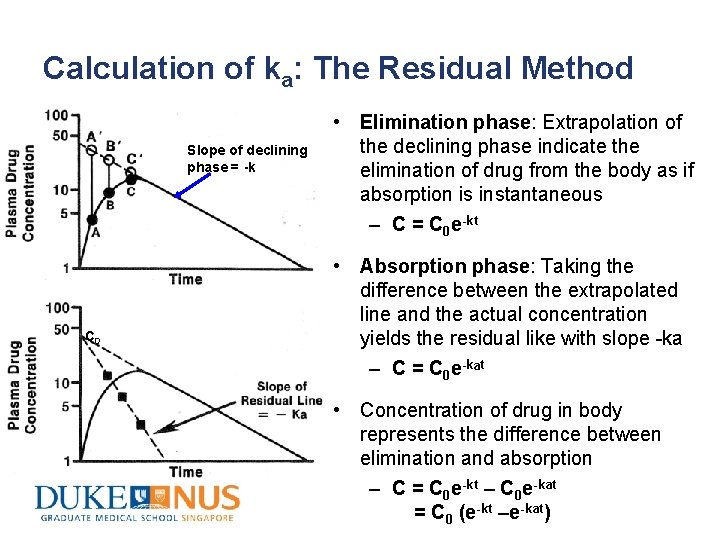
Calculation of ka: The Residual Method Slope of declining phase = -k C 0 • Elimination phase: Extrapolation of the declining phase indicate the elimination of drug from the body as if absorption is instantaneous – C = C 0 e-kt • Absorption phase: Taking the difference between the extrapolated line and the actual concentration yields the residual like with slope -ka – C = C 0 e-kat • Concentration of drug in body represents the difference between elimination and absorption – C = C 0 e-kt – C 0 e-kat = C 0 (e-kt –e-kat)

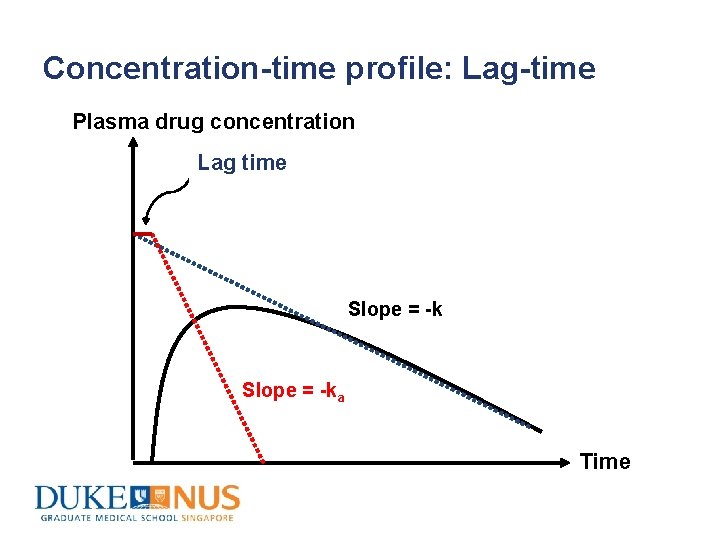
Concentration-time profile: Lag-time Plasma drug concentration Lag time Slope = -ka Time

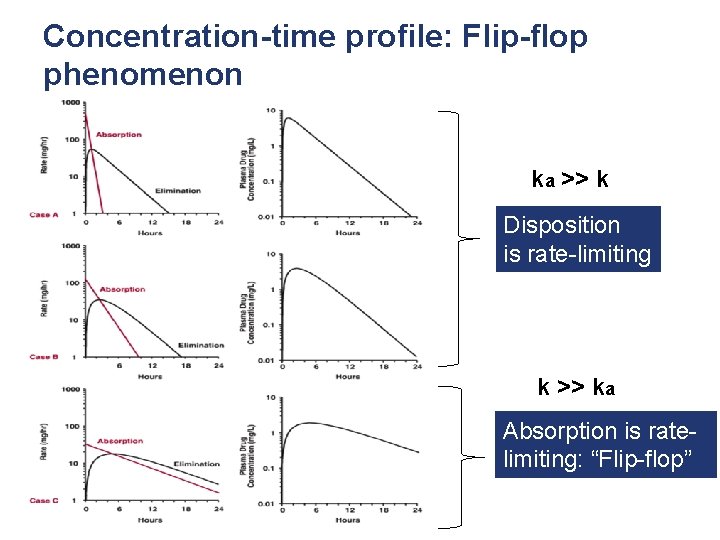
Concentration-time profile: Flip-flop phenomenon ka >> k Disposition is rate-limiting k >> ka Absorption is ratelimiting: “Flip-flop”

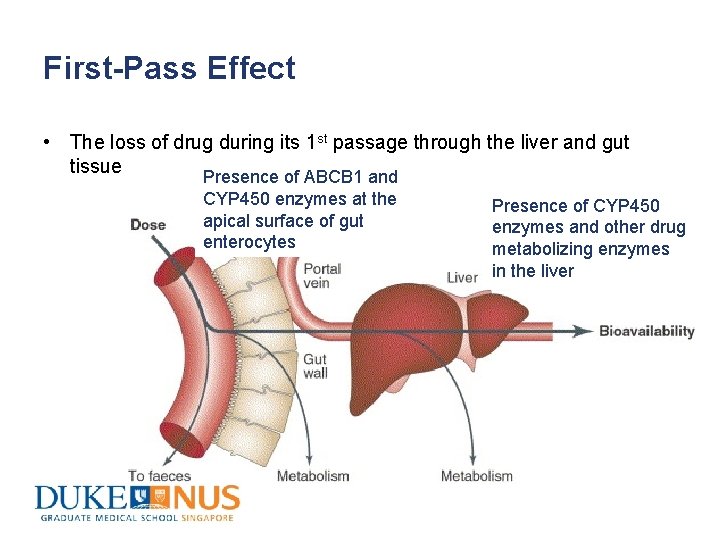
First-Pass Effect • The loss of drug during its 1 st passage through the liver and gut tissue Presence of ABCB 1 and CYP 450 enzymes at the apical surface of gut enterocytes Presence of CYP 450 enzymes and other drug metabolizing enzymes in the liver

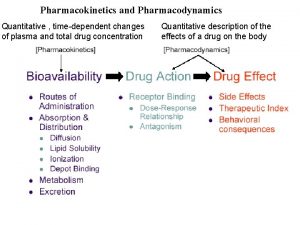
Bioavailability (F) • Definition – The rate and extent to which the active moiety is absorbed from a drug product and becomes available at the site of action. • Provides an estimate of the relative fraction of the orally administered dose that is absorbed into the systemic circulation • For drugs administered intravenously, F = 1

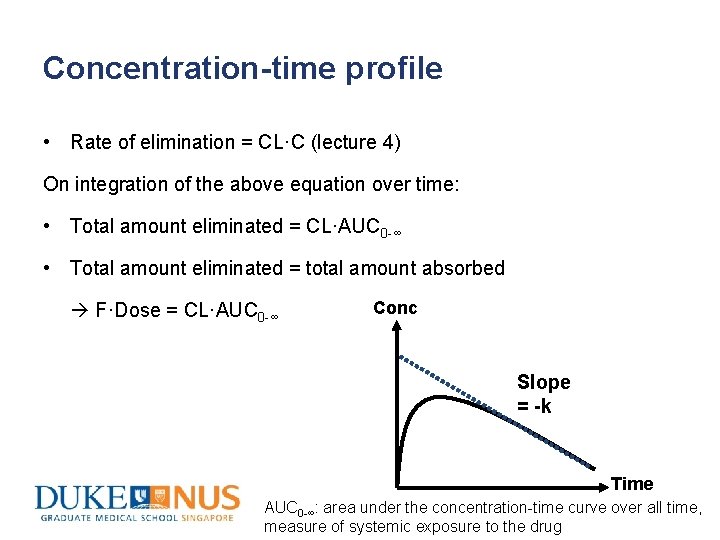
Concentration-time profile • Rate of elimination = CL·C (lecture 4) On integration of the above equation over time: • Total amount eliminated = CL·AUC 0 -∞ • Total amount eliminated = total amount absorbed F·Dose = CL·AUC 0 -∞ Conc Slope = -k Time AUC 0 -∞: area under the concentration-time curve over all time, measure of systemic exposure to the drug

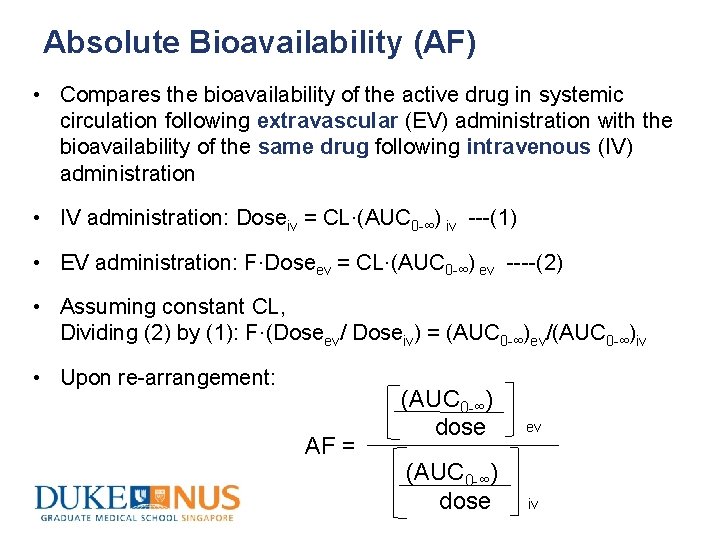
Absolute Bioavailability (AF) • Compares the bioavailability of the active drug in systemic circulation following extravascular (EV) administration with the bioavailability of the same drug following intravenous (IV) administration • IV administration: Doseiv = CL·(AUC 0 -∞) iv ---(1) • EV administration: F·Doseev = CL·(AUC 0 -∞) ev ----(2) • Assuming constant CL, Dividing (2) by (1): F·(Doseev/ Doseiv) = (AUC 0 -∞)ev/(AUC 0 -∞)iv • Upon re-arrangement: AF = (AUC 0 -∞) dose ev iv

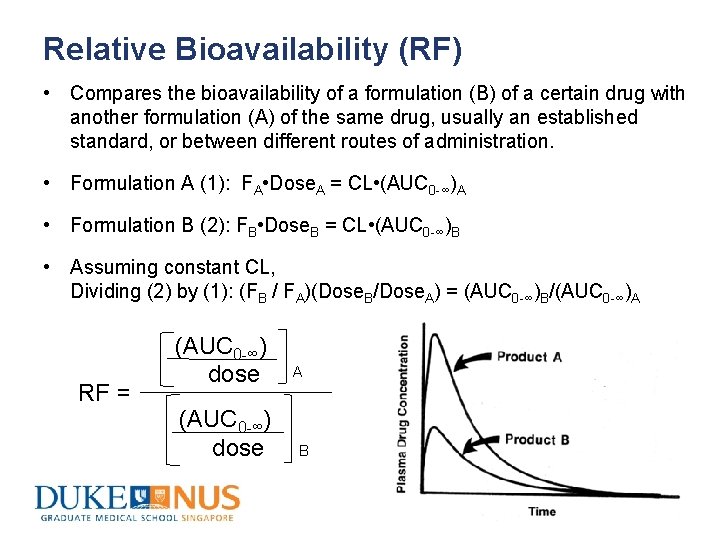
Relative Bioavailability (RF) • Compares the bioavailability of a formulation (B) of a certain drug with another formulation (A) of the same drug, usually an established standard, or between different routes of administration. • Formulation A (1): FA • Dose. A = CL • (AUC 0 -∞)A • Formulation B (2): FB • Dose. B = CL • (AUC 0 -∞)B • Assuming constant CL, Dividing (2) by (1): (FB / FA)(Dose. B/Dose. A) = (AUC 0 -∞)B/(AUC 0 -∞)A RF = (AUC 0 -∞) dose A B

Bioavailability (BA) • Bioavailability studies - a requirement by the US FDA for all new drug applications – ka as direct measure of the rate of absorption – Cmax and tmax as indirect measures of the rate of absorption – Extent of absorption is determined by AF/RF • BA data can also indirectly provide information about the drug permeability and the influence of presystemic metabolizing enzymes and/or transporters (e. g. , p-glycoprotein).

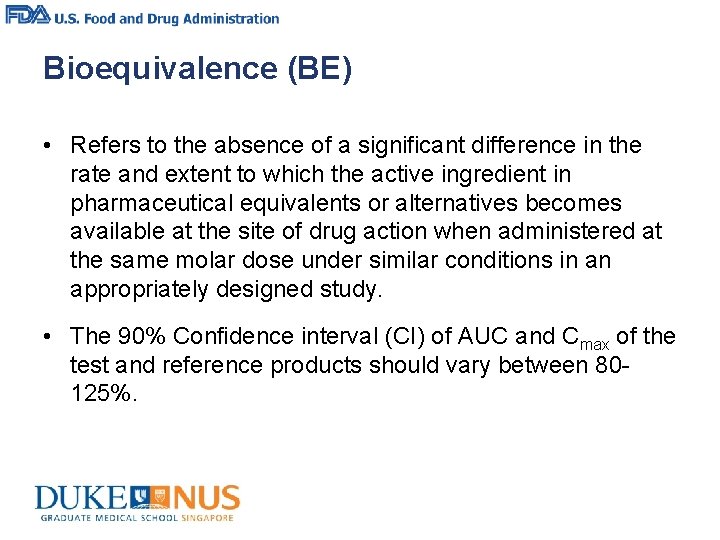
Bioequivalence (BE) • Refers to the absence of a significant difference in the rate and extent to which the active ingredient in pharmaceutical equivalents or alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study. • The 90% Confidence interval (CI) of AUC and Cmax of the test and reference products should vary between 80125%.

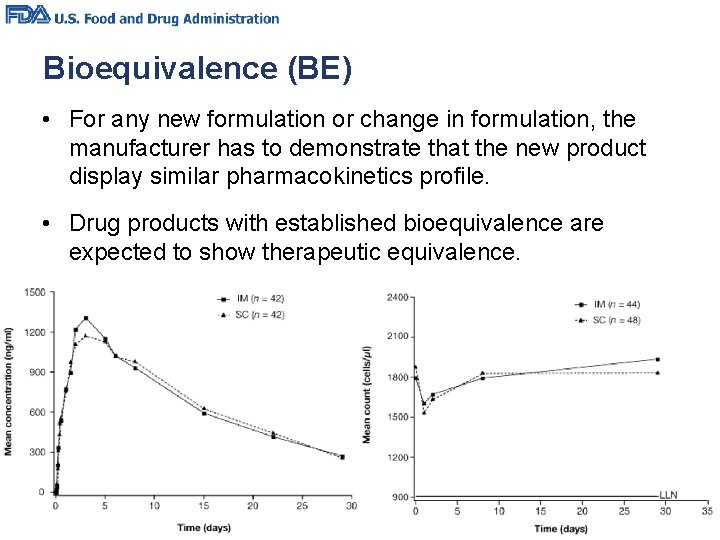
Bioequivalence (BE) • For any new formulation or change in formulation, the manufacturer has to demonstrate that the new product display similar pharmacokinetics profile. • Drug products with established bioequivalence are expected to show therapeutic equivalence.

Methods to demonstrate Bioavailability (BA) & Bioequivalence (BE) • Several in vivo and in vitro methods can be used to measure BA and to establish BE: – Pharmacokinetic > pharmacodynamic > clinical > in vitro studies. • Studies of BA and BE frequently rely on pharmacokinetic measures (AUC and Cmax) that reflect the level of systemic exposure. • in vivo BA and BE studies can be waived off for drugs exhibiting high solubility and permeability

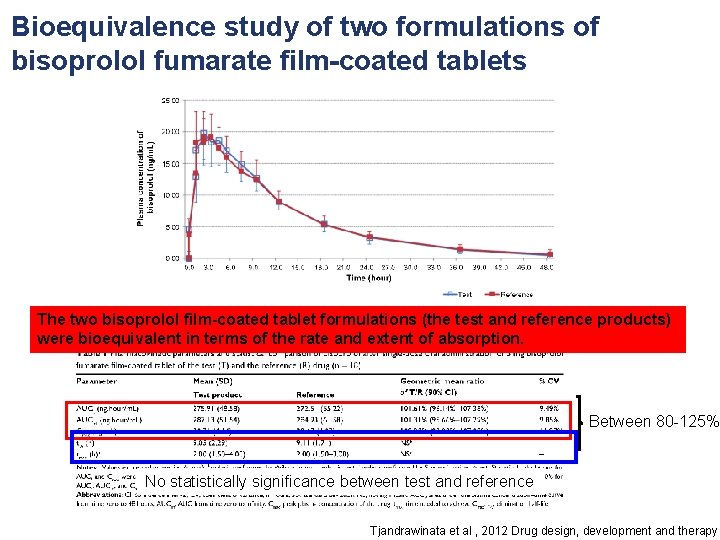
Bioequivalence study of two formulations of bisoprolol fumarate film-coated tablets The two bisoprolol film-coated tablet formulations (the test and reference products) were bioequivalent in terms of the rate and extent of absorption. Between 80 -125% No statistically significance between test and reference Tjandrawinata et al , 2012 Drug design, development and therapy

References • Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications Malcolm Rowland, Thomas N Tozer • Concepts in Clinical Pharmacokinetics: A Self. Instructional Course, Joseph T. Dipiro • Applied Biopharmaceutics & Pharmacokinetics (Fifth Edition), Leon Shargel, Susanna Wu-Pong, Andrew BC Yu

Thank you www. duke-nus. edu. sg
 One compartment open model extravascular administration
One compartment open model extravascular administration Extravascular administration example
Extravascular administration example Drug metabolism and pharmacokinetics
Drug metabolism and pharmacokinetics Extravascular sensor
Extravascular sensor What are the different routes of drug administration
What are the different routes of drug administration Intrademal
Intrademal Topical routes
Topical routes First pass effect in pharmacology
First pass effect in pharmacology First pass effect
First pass effect Non parenteral route
Non parenteral route Angesid
Angesid Intrathecal route advantages and disadvantages
Intrathecal route advantages and disadvantages Intradermal drug administration
Intradermal drug administration Jordan food and drug administration
Jordan food and drug administration Accidental adulteration definition
Accidental adulteration definition What is pharmacokinetics
What is pharmacokinetics Define relative bioavailability
Define relative bioavailability Basic principles of pharmacokinetics
Basic principles of pharmacokinetics Pharmacokinetics vs pharmacodynamics
Pharmacokinetics vs pharmacodynamics Objectives of pharmacokinetics
Objectives of pharmacokinetics Characteristics of non linear pharmacokinetics
Characteristics of non linear pharmacokinetics Non linear pharmacokinetics
Non linear pharmacokinetics Non linear pharmacokinetics
Non linear pharmacokinetics Multi compartment model pharmacokinetics
Multi compartment model pharmacokinetics Factors affecting drug metabolism slideshare
Factors affecting drug metabolism slideshare Pharmacokinetic definition
Pharmacokinetic definition Nebido half life
Nebido half life Introduction to biopharmaceutics and pharmacokinetics
Introduction to biopharmaceutics and pharmacokinetics