Pharmaceutical TNCs Glaxo Smith Kline Health matters in




















- Slides: 25

Pharmaceutical TNCs Glaxo. Smith. Kline

Health matters in a globalising world • TNCs – Transnational Corporations are companies that operate in at least two countries (and often many more). • Headquarters and research facilities tend to be located in MEDCs whilst manufacturing plants have increasingly been located in LEDCs to take advantage of cheaper labour costs.

Pharmaceutical TNCs Glaxo. Smith. Kline is the UK’s largest pharmaceutical company and one of the biggest in the world. It had sales in excess of $37 million in 2006. Where might you see Glaxo. Smith. Klein products on the High Street? Apart from drugs, Glaxo. Smith. Kline owns products such as Aquafresh toothpaste, Beechams Cold & Flu and Lucozade.


Glaxo. Smith. Kline • • Mission: to improve the quality of human life by enabling people to do more, feel better and live longer. Research based pharmaceutical company. Employs over 100, 000 people in 117 countries Every hour Glaxo spends more than £ 300, 000 to find new medicines. Only pharmaceutical to tackle three ‘priority’ diseases identified by the WHO: HIV/AIDS, tuberculosis, malaria. In 2006 Glaxo shipped 206 million tablets of their HIV treatments (Combivir and Epivir) to developing countries Many consumer brands are household names: Ribena, Horlicks, Lucozade, Aquafresh, Sensodyne, Panadol.

• GSK was 1 of the 39 pharmaceutical companies involved in the South Africa legal case. • Glaxo. Smith. Kline, has now changed its tactics completely and has granted permission (called a voluntary licence) to major South African generics producer Aspen, to share the rights to their drugs without charge. • http: //www. gsk. com/infocus/world-aidsday. htm video clip/propaganda? !/good PR

Branded and generic drugs • The generic name of a drug is its chemical description. • Brand names of drugs are the ones people recognise due to marketing and they are often shorter and catchier than the generic names. • Branded drugs are 3 to 30 times more expensive than generic drugs. Have you heard of Fluoxetine hydrochloride? How about Prozac? Prozac is the branded drug. Fluoxetine hydrochloride is the generic name.

The Name Game • • A pharmaceutical company discovers a new generic drug to treat or prevent a condition, They put it through a series of clinical trials in order to gain approval for marketing from the Medicines and Healthcare products Regulatory Agency (MHRA). the MHRA approves the drug and gives it a licence. the pharmaceutical company can then market the generic medicine under a brand name. The company then has exclusive rights to market the medicine for the licensed uses for a certain period of time, usually about 10 to 12 years. This is known as a patent, and allows the drug company to recoup the costs of research and development of the new medicine, before other drug companies are allowed to produce it as well. Other drug companies are likely to be able to produce and sell the medicine at a cheaper rate, because the research and development has already been done. Once a patent expires, other drug companies then have the right to manufacture and market the generic drug. However, they must market it under a different brand name, or under its generic name.

• For example, sildenafil (Viagra) is still under patent and so can currently only be marketed by Pfizer to treat impotence. Once the patent expires, we can expect to see other pharmaceutical companies marketing potentially cheaper versions of the generic medicine sildenafil, either under different brand names, or simply as the generic sildenafil. • Ibuprofen on the other hand is a much older medicine and can already be bought under various different brand names, eg Nurofen (made by Reckitt Benckiser), and Anadin ultra (made by Wyeth Consumer Healthcare), to name but a few. All of these contain ibuprofen as the generic medicine. Ibuprofen can also be bought simply as ibuprofen tablets, made by various different manufacturers who market it without a brand name.

Branded vs. generic • • Generic drugs are cheaper. The NHS could save up to £ 85 million by prescribing generic statin drugs to patients with high cholesterol. A which report sites medicines such as paracetamol and ibuprofen can be bought for a fraction of the price of branded medicines if shoppers seek out less prominent labels. It cites Panadol, which costs £ 1. 85 for 16 tablets but contains the same amount of the active ingredient as Sainsbury's ownbrand paracetamol, which costs 26 p for the same size pack. Drug manufactures put large amounts of money into research and development of new and existing drugs and need to recuperate this through sales. • • Epilepsy medication: Anecdotal studies and small scale research report that switching epilepsy suffers from branded medicine to generic medicine can mean seizures return. The placebo effect. Marketing makes people think branded drugs work better, so they believe they do.

How do Pharmaceutical TNCs develop new drugs? • It is estimated that new drug costs $500 million to bring to market. • Most money is spent targeting diseases of affluence as MEDCs can pay high sums for treatments. • Much less money is spent on those diseases like HIV / AIDS and malaria that affect millions in LEDCs. • This figure includes the R&D in labs, clinical trials, marketing (especially to doctors) etc. • Patents make it illegal to copy the drug and for rival companies to make a generic version for 20 years.

Brief History of AIDS drugs in Africa • In 1996, HAART - an effective combination therapy that delays the onset of AIDS - became available in MEDC’s. Within four years, death rates for people with HIV/AIDS in developed countries had dropped by 84%. • At a cost of US$10, 000 -15, 000 person per year, these antiretroviral drugs (ARVs) were far too expensive for the majority of HIV infected people in resource poor countries. • Five years after HAART therapy was introduced in the West, fewer than 8, 000 people in sub-Saharan Africa were receiving the life-saving drugs. • Big Pharma companies refused to lower prices saying there would be no money for research and development (R&D), no innovation, and thus more and more people would die from AIDS and other deadly diseases.

In 2001 an attempt was made by thirty-nine major pharmaceutical companies to prosecute the South African government for passing a law that allowed easy production and importation of generics.

Brief History of AIDS drugs in Africa • In the Year 2000 an Indian pharmaceutical company called Cipla started to produce generic antiretrovirals that were exactly the same as brand versions made by large pharmaceutical companies, but significantly cheaper. • This sparked a price war between branded and generic drug makers, which forced the large pharmaceutical companies to lower the price of their AIDS drugs. • This competition, coupled with pressure from activists, organizations - such as the Clinton Foundation - and governments of poor countries with severe AIDS epidemics, dramatically reduced the price of ARVs for developing countries. • By the middle of 2001, triple combination therapy was available from Indian generic manufacturers for as little as $295. By 2007 the most common antiretroviral combination (3 TC/d 4 T/NVP) available for only US$87 per patient per year

GSK and AIDS • The global community is aware of the scale of the problem. For its part, the pharmaceutical industry is working hard to find new and more effective treatments for HIV, which can lead to AIDS. • A lot of emphasis is placed on anti-retroviral treatments. • Glaxo. Smith. Kline is helping with an array of initiatives including improving the convenience of delivering anti-retroviral therapy, developing molecules that address drug-resistant HIV. • In addition, the company is carrying out research into a vaccine against HIV infection. • The search is one for a cure, although there is a huge local demand as well as an international demand to help reduce the spread and impact of HIV and AIDS. • Various community-based groups provide a wide range of information, counselling, care and other support services. These groups form the backbone of the fight against HIV/AIDS in many countries where governments are unable or unwilling to combat the effects of the epidemic. • GSK works with these groups through its Positive Action programme.

Pharmaceutical companies: heroes or villains? GSK is directly responsible for numerous deaths due to its patents held, and drug pricing strategies GSK is at the forefront of developing immunisations and treatments for diseases in developing countries. Profits are ploughed back in to research. HIV AIDS treatments are sold at cost in many countries GSK share price is declining, they are not reaching the expected 55 p and profits are slightly down from the £ 22. 7 billion made last year. • Who might have said the statement, does it make GSK a hero or a villain?

Globalisation: Medical Imports and Medical Exports

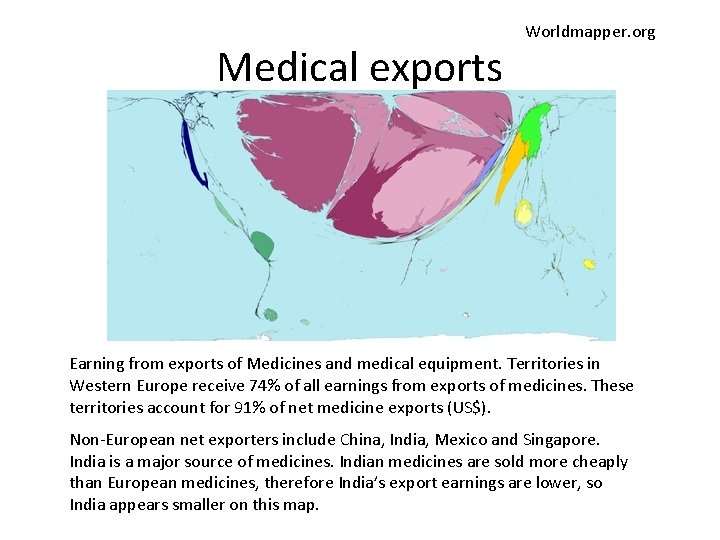
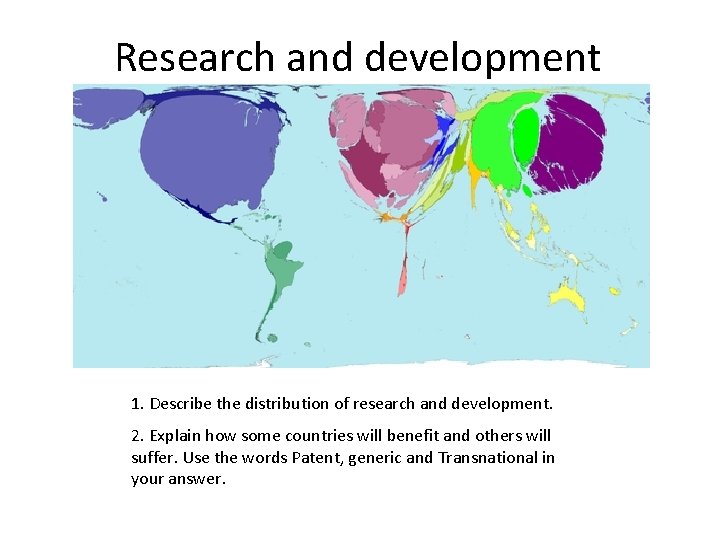
Medical exports Worldmapper. org Earning from exports of Medicines and medical equipment. Territories in Western Europe receive 74% of all earnings from exports of medicines. These territories account for 91% of net medicine exports (US$). Non-European net exporters include China, India, Mexico and Singapore. India is a major source of medicines. Indian medicines are sold more cheaply than European medicines, therefore India’s export earnings are lower, so India appears smaller on this map.

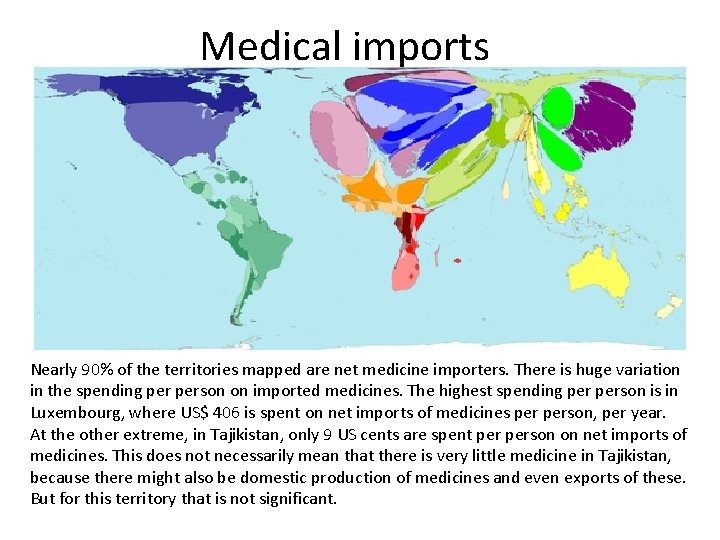
Medical imports Nearly 90% of the territories mapped are net medicine importers. There is huge variation in the spending person on imported medicines. The highest spending person is in Luxembourg, where US$ 406 is spent on net imports of medicines person, per year. At the other extreme, in Tajikistan, only 9 US cents are spent person on net imports of medicines. This does not necessarily mean that there is very little medicine in Tajikistan, because there might also be domestic production of medicines and even exports of these. But for this territory that is not significant.


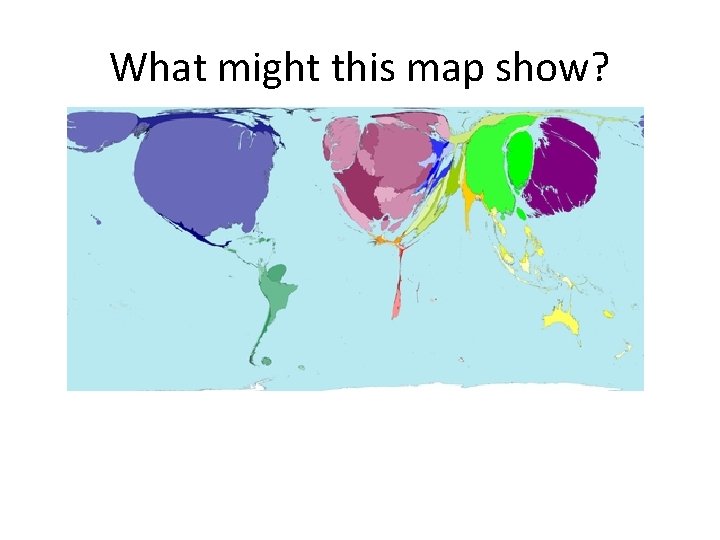
What might this map show?

Research and development 1. Describe the distribution of research and development. 2. Explain how some countries will benefit and others will suffer. Use the words Patent, generic and Transnational in your answer.

Research and development • In 2002, US$289 billion was spent on research and development in the United States; in the same year there was practically no research and development spending in Angola. It is thus unsurprising that the number of patents granted and the value of royalty and license fees received are also vastly different between these places.

• Many people, most of them in tropical countries of the Third World, die of preventable, curable diseases. … Malaria, tuberculosis, acute lowerrespiratory infections—in 1998, these claimed 6. 1 million lives. People died because the drugs to treat those illnesses are nonexistent or are no longer effective. They died because it doesn’t pay to keep them alive. • — Ken Silverstein, Millions for Viagra, Pennies for Diseases of the Poor, The Nation, July 19, 1999

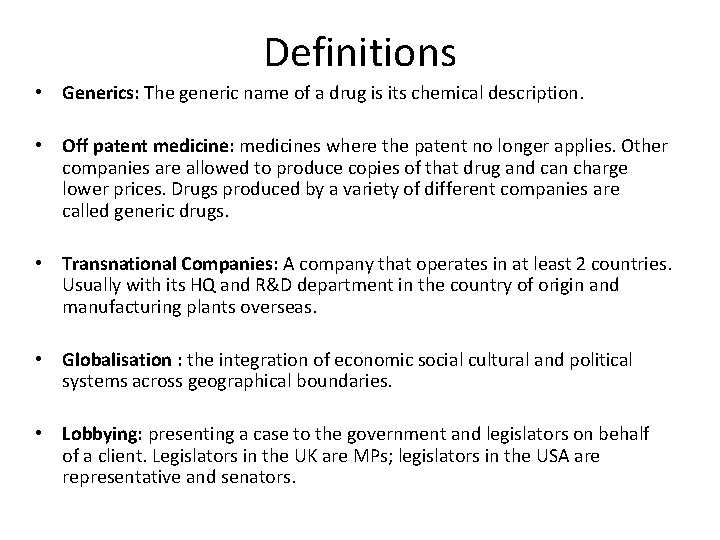
Definitions • Generics: The generic name of a drug is its chemical description. • Off patent medicine: medicines where the patent no longer applies. Other companies are allowed to produce copies of that drug and can charge lower prices. Drugs produced by a variety of different companies are called generic drugs. • Transnational Companies: A company that operates in at least 2 countries. Usually with its HQ and R&D department in the country of origin and manufacturing plants overseas. • Globalisation : the integration of economic social cultural and political systems across geographical boundaries. • Lobbying: presenting a case to the government and legislators on behalf of a client. Legislators in the UK are MPs; legislators in the USA are representative and senators.
 Top 100 foundations
Top 100 foundations Alemtuzumab
Alemtuzumab Tns or tncs
Tns or tncs Glaxo parma
Glaxo parma Nancy kline 10 components thinking environment
Nancy kline 10 components thinking environment Chief franz kline
Chief franz kline Query kline
Query kline Nathan kline institute
Nathan kline institute Kevin kline sql
Kevin kline sql Health matters paragraph
Health matters paragraph Health matters amesbury
Health matters amesbury Ralph smith public health
Ralph smith public health Knowledge matters parking sim answers
Knowledge matters parking sim answers Inner well being
Inner well being Says means matters
Says means matters Race matters for juvenile justice
Race matters for juvenile justice Nothing matters question tag
Nothing matters question tag Why punctuation matters
Why punctuation matters Why productivity matters
Why productivity matters Care matters nationwide
Care matters nationwide Money matters topic
Money matters topic Performance matters dadeschools
Performance matters dadeschools Energy matters dvd cover
Energy matters dvd cover Standard data quality dimensions kpmg
Standard data quality dimensions kpmg Food hygiene matters
Food hygiene matters Cranial nerves classification
Cranial nerves classification