Percutaneous left atrial appendage closure versus novel anticoagulation



- Slides: 21

Percutaneous left atrial appendage closure versus novel anticoagulation agents in high-risk atrial fibrillation patients (PRAGUE-17 study) Pavel Osmancik, Dalibor Herman, Petr Neuzil, Pavel Hala, Milos Taborsky, Petr Kala, Martin Poloczek, Josef Stasek, Ludek Haman, Marian Branny, Jan Chovancik, Pavel Cervinka, Jiri Holy, Tomas Kovarnik, David Zemanek, Stepan Havranek, Vlastimil Vancura, Richard Rokyta, Petr Peichl, Petr Tousek, Veronika Lekesova, Jiri Jarkovsky, Martina Novackova, Klara Benesova, Petr Widimsky & Vivek Y. Reddy IIIrd Internal – Cardiology Clinic, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic

Conflicts of Interest • • • • Pavel Osmancik: Bayer & Abbott – Speaking honoraria Dalibor Herman: None Petr Neuzil: None Milos Taborsky: Bayer and Pfizer – Advisory board Martin Poloczek: Abbott – Speaking honoraria Petr Kala: Bayer – Speaker bureau/Advisory board; Boston Scientific – Advisory board Josef Stasek: None Ludek Haman: Pfizer – Speaking honoraria Jan Chovancik: None Pavel Cervinka: None Tomas Kovarnik: None Vlastimil Vancura: None Petr Peichl: None Petr Widimsky: Bayer, Pfizer, & Boehringer Ingelheim – Honoraria Vivek Y. Reddy: Abbott & Boston Scientific – Consulting income and Grant support

Introduction • PROTECT-AF & PREVAIL: • Left atrial appendage closure (LAAC) was non-infeior to vitamin K antagonists (VKA) for ischemic stroke and systemic embolism • LAAC was superior for intracranial hemorrhage (and associated reduction in cardiovascular mortality) • ROCKET-AF, RE-LY, ARISTOTLE & ENGAGE-AF • Non-VKA oral anticoagulants (NOACs) are associated with less intracranial hemorrhage, all-stroke, and mortality Ø Since the primary benefit of LAAC over VKA was reduced ICH, which is also reduced with NOACs, the relative efficacy and safety of LAAC vs NOACs are unknown.

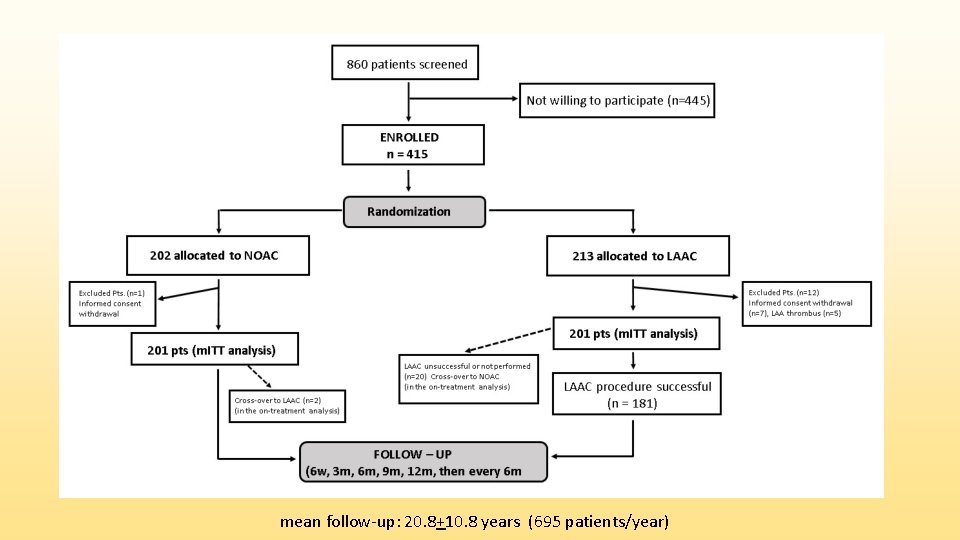
Study design • PRAGUE-17 (NCT 02426944) was an investigator-initiated, multicenter, prospective, open-label, randomized non-inferiority trial • Goal: to compare LAAC with NOAC in high risk AF patients • Enrollment period: October 2015 to January 2019 • Conducted in 10 Cardiac Centers in the Czech Republic, coordinated by Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic

Inclusion criteria • Non-valvular atrial fibrillation + one of the following: I. History of bleeding requiring intervention or hospitalization, or II. History of a cardioembolic event while taking anticoagulation, or III. CHA 2 DS 2 -VASc ≥ 3 & HAS-BLED ≥ 2

Exclusion criteria • • • Mechanical valve prosthesis, or mitral stenosis Comorbidities other than AF mandating anticoagulation Patent foramen ovale with large atrial septal aneurysm Mobile aortic plaque Symptomatic carotid arterial atherosclerosis Pericardial effusion > 10 mm Clinically significant bleeding within 30 days Stroke or other cardioembolic event within the 30 days Acute coronary syndrome within the 90 days Creatinine clearance < 30 ml/min If randomized to LAAC, the presence of an LA/LAA thrombus

Randomization • Randomization performed using a centralized computer system: • LAAC or NOAC in a 1: 1 ratio • Block sizes varying between 18– 22 patients • Stratified by center to ensure comparable CHA 2 DS 2 VASc scores • NOAC group: • Rivaroxaban, Apixaban, or Dabigatran at manufacturer-recommended dose • Apixaban preferred • LAAC group: • Amulet device (Abbott Inc, St. Paul, MN) or • Watchman / Watchman-FLX device (Boston Scientific Inc, St. Paul, MN)

Antithrombotic treatment in the LAAC group • Default Strategy: • DAPT x 3 months: aspirin (100 mg/day) + clopidogrel (75 mg/day) • TEE (3 months): clopidogrel withdrawn • aspirin continued indefinitely • Regimen could be individualized • High bleeding risk patients: DAPT could be shortened to 6 weeks • Very high thrombotic risk patients: Ø NOAC substitution for DAPT x 3 months, or Ø VKAs x 6 weeks DAPT x 6 weeks, or Ø NOACs x 6 weeks DAPT x 4. 5 months

Study outcomes Primary Endpoint: a composite of : (1) Stroke or transient ischemic attack (TIA) (2) Systemic embolism (3) Clinically-significant bleeding * (4) Cardiovascular death, or (5) significant peri-procedural or device-related complication. * Clinically-significant bleeding = ISTH major or non-major clinically-significant bleeding. Major bleeding: Either a decrease in hemoglobin ≥ 2. 0 g/d. L over 24 -hrs, transfusion of ≥ 2 units of packed red cells, bleeding at a critical site (intracranial, intraspinal, intraocular, pericardial, intramuscular with compartment syndrome or retroperitoneal), or fatal bleeding Non-major bleeding is defined as bleeding requiring hospitalization or an invasive procedure, but not meeting ISTH major criteria

Statistics • Primary hypothesis: LAAC is noninferior to NOAC for the primary endpoint • Primary analysis = modified intention-to–treat • Also secondary analyses: on-treatment & per-protocol • Power calculation: estimated annual occurrence of primary endpoint is 13% for NOACs and 10% for LAAC, to achieve 80% power at a twosided alpha level of 0. 05 for a noninferiority margin of 5% (or 1. 47 as odds ratio), 396 subjects needed to enroll

mean follow-up: 20. 8+10. 8 years (695 patients/year)

Baseline characteristics and risk factors Age (years) Male gender (%) AF type Paroxysmal (%) Persistent (%) LS persistent (%) Permanent (%) CHA 2 DS 2 -VASc > 6 (%) HAS-BLED Heart failure (%) Hypertension (%) Diabetes mellitus (%) History of cardioembolic event (%) History of MI (%) History of bleeding/bleeding predisposition NOAC (n = 201) LAAC (n = 201) 73. 2 ± 7. 2 130 (64. 7%) 67 (33. 3%) 46 (22. 9%) 16 (8. 0%) 72 (35. 8%) 4. 7 ± 1. 5 54 (26. 9%) 3. 0 ± 0. 9 90 (44. 8%) 186 (92. 5%) 90 (44. 8%) 69 (34. 3%) 39 (19. 4%) 95 (47. 3%) 73. 4 ± 6. 7 134 (66. 7%) 53 (26. 4%) 47 (23. 4%) 18 (9. 0%) 83 (41. 3%) 4. 7 ± 1. 5 56 (27. 9%) 3. 1 ± 0. 9 88 (43. 8%) 186 (92. 5%) 73 (36. 3%) 30 (14. 9%) 109 (54. 2%)

Treatment characteristics – LAAC arm • 201 patients allocated to the LAAC arm • 14 (7. 0%) did not undergo the procedure because of either anatomical considerations (large LAA; n=3), pericardial effusion (n=1), suspicion of infective endocarditis (n=1), or patient refusal (n=9). [12 pts crossed over to NOAC] • 187 patients underwent the procedure; successful in 96. 8% (181/187) of procedure attempts, or in 90% (181/201) of patients assigned to LAAC • Implanted devices: Amulet-61. 3%, Watchman-35. 9% or Watchman-Flex-2. 8%. • DAPT in 82% after LAAC. • Complications: in 9 pts (4. 8%) including one procedure-related death (groin hematoma, vascular surgery, MI/death), and one device-related death (late pericardial tamponade)

Treatment characteristics – NOAC arm • Apixaban - 192 patients (95. 5%): • 2 x 5 mg in 159 (79. 1%) patients • 2 x 2. 5 mg in 33 (16. 4%) patients • Dabigatran - 8 patients • 2 x 150 mg in 7 (3. 5%) patients • 2 x 110 mg in 1 (0. 5%) patient • Rivaroxaban – 1 patient (0. 5%) • 1 x 20 mg daily

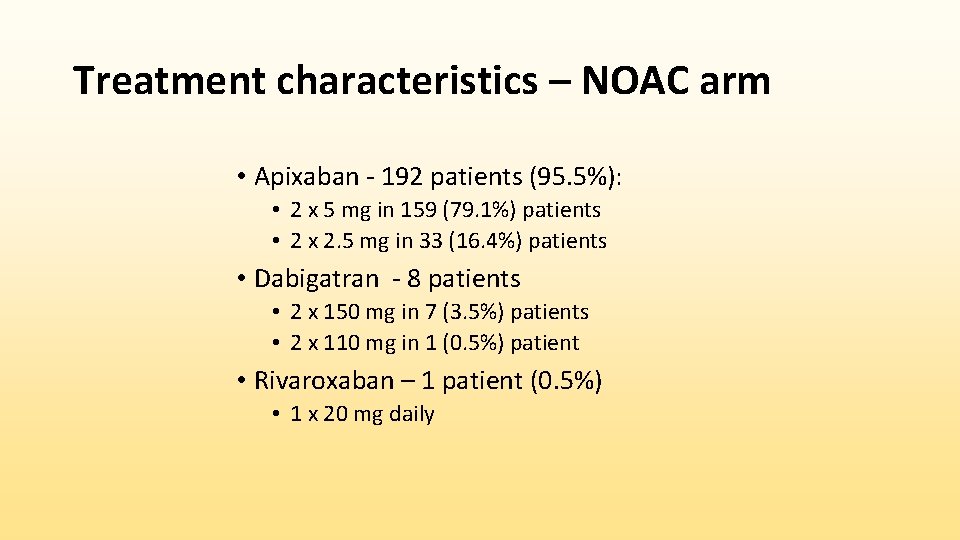
Cumulative incidence function (CIF) for primary study endpoint in intention-to-treat populations Gray´s test: p = 0. 44 Subdistribution HR: 0. 84 (0. 53 – 1. 31), p = 0. 44 Non-inferiority: p = 0. 004 *Number of patients who are free of any event is supplemented (in brackets) by number of patients with competing risk up to given time.

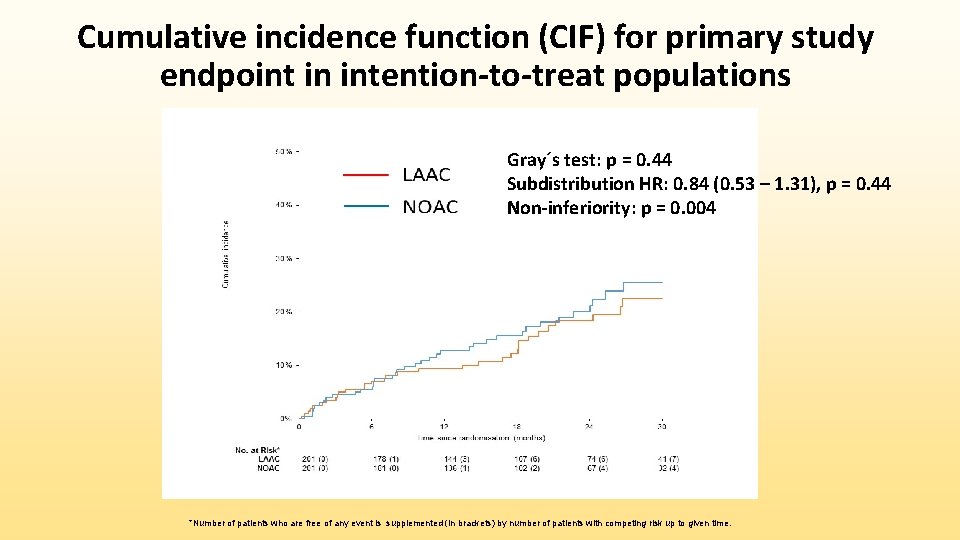
Cumulative incidence function for all-stroke/TIA and cardiovascular death in ITT populations Gray´s test: p= 0. 99 Subdistribution HR: 0. 99 (0. 39 - 2. 50) Gray´s test: p = 0. 46 Subdistribution HR: 0. 75 (0. 34 – 1. 62) *Number of patients who are free of any event is supplemented (in brackets) by number of patients with competing risk up to given time.

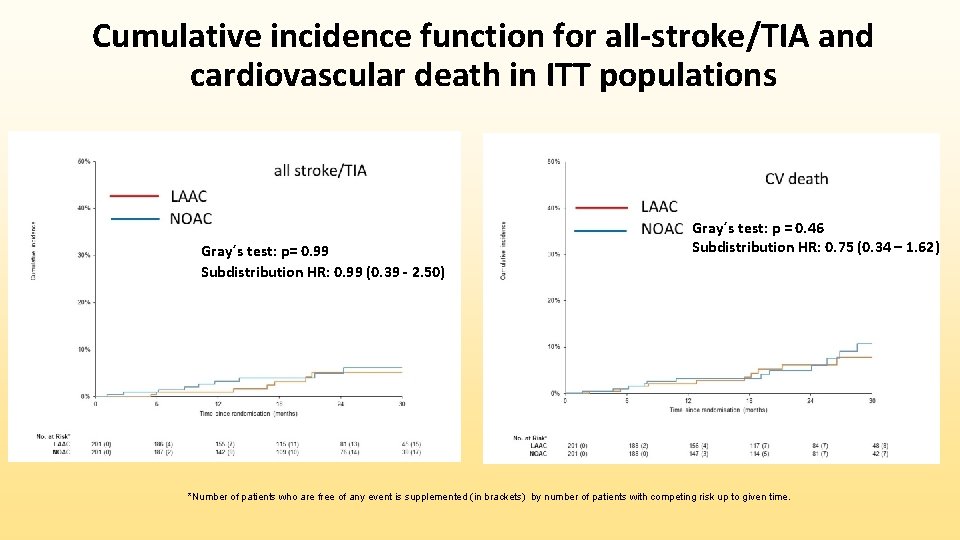
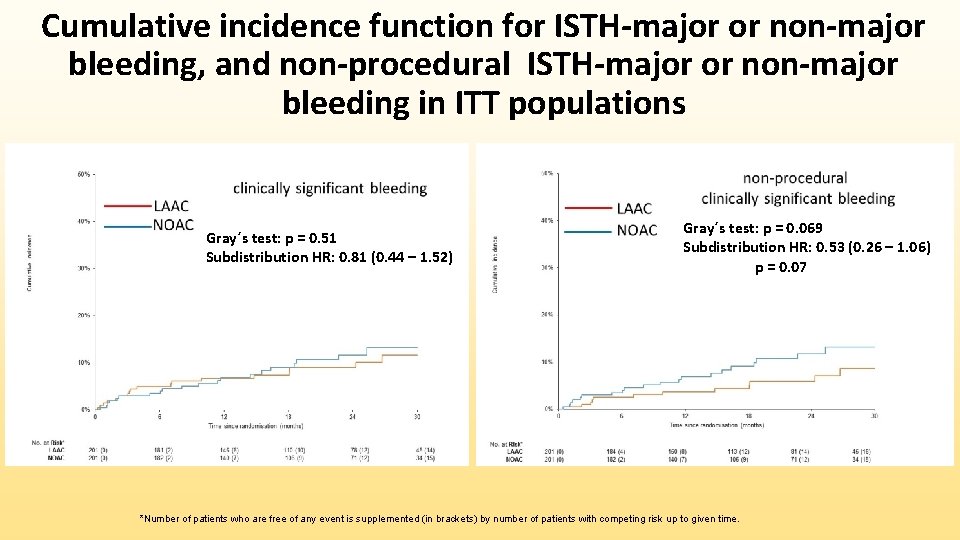
Cumulative incidence function for ISTH-major or non-major bleeding, and non-procedural ISTH-major or non-major bleeding in ITT populations Gray´s test: p = 0. 51 Subdistribution HR: 0. 81 (0. 44 – 1. 52) Gray´s test: p = 0. 069 Subdistribution HR: 0. 53 (0. 26 – 1. 06) p = 0. 07 *Number of patients who are free of any event is supplemented (in brackets) by number of patients with competing risk up to given time.

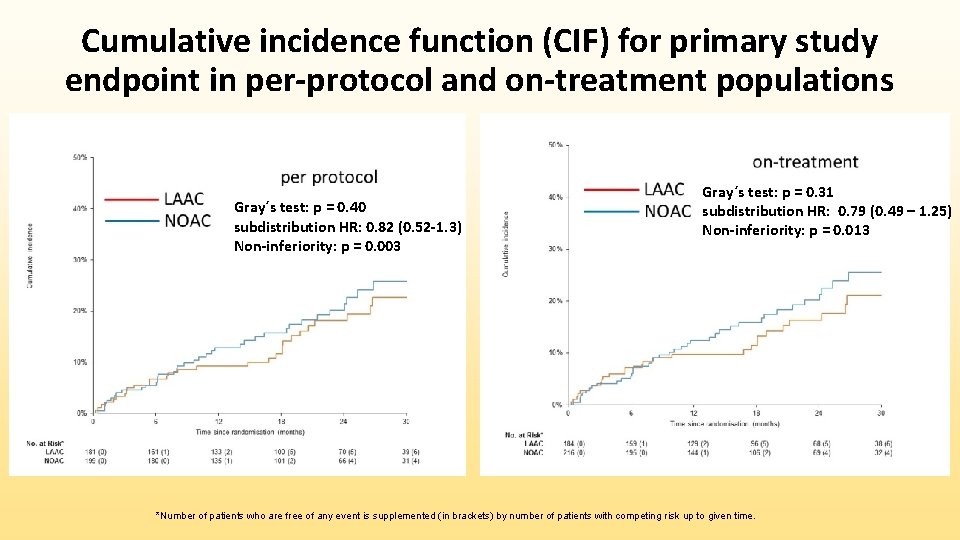
Cumulative incidence function (CIF) for primary study endpoint in per-protocol and on-treatment populations Gray´s test: p = 0. 40 subdistribution HR: 0. 82 (0. 52 -1. 3) Non-inferiority: p = 0. 003 Gray´s test: p = 0. 31 subdistribution HR: 0. 79 (0. 49 – 1. 25) Non-inferiority: p = 0. 013 *Number of patients who are free of any event is supplemented (in brackets) by number of patients with competing risk up to given time.

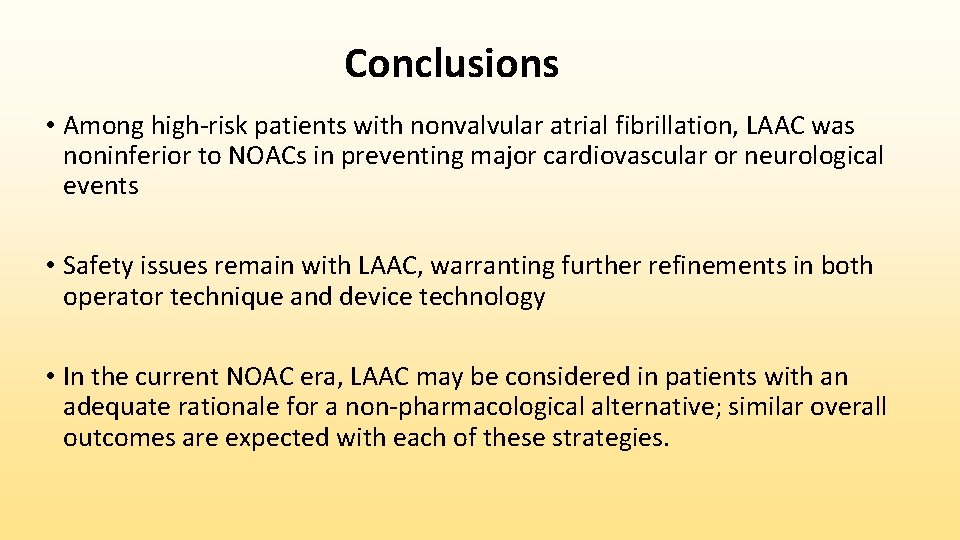
Conclusions • Among high-risk patients with nonvalvular atrial fibrillation, LAAC was noninferior to NOACs in preventing major cardiovascular or neurological events • Safety issues remain with LAAC, warranting further refinements in both operator technique and device technology • In the current NOAC era, LAAC may be considered in patients with an adequate rationale for a non-pharmacological alternative; similar overall outcomes are expected with each of these strategies.

Study limitations • Insufficiently powered to evaluate the relative differences in the individual components of the primary endpoint (stroke, death, bleeding) • Despite substantial follow-up (20. 8± 10. 8 months), additional followup is needed to determine long-term differences between groups

Acknowledgements LIST OF INVESTIGATORS: • Cardiocenter, Third Faculty of Medicine, Charles University Prague and University Hospital Kralovske Vinohrady, Prague, Czech Republic : Pavel Osmancik, MD, Ph. D, Dalibor Herman, MD, Ph. D, Petr Tousek, MD, Ph. D, Petr Widimsky, MD, Dr. Sc • Cardiocenter, Department of Cardiology, Na Homolce Hospital, Prague, Czech Republic : Vivek Y. Reddy, MD, Petr Neuzil, MD, CSc, Pavel Hala, MD, Veronika Lekesova, MD • Cardiocenter, Dept. of Cardiology, University Hospital Olomouc, Czech Republic : Milos Taborsky, MD, CSc, Irena Opavska, Mgr, Miloslav Spacek MD • Clinic of Cardiology, Masaryk University and University Hospital Brno, Czech Republi c: Petr Kala, MD, Ph. D, Martin Poloczek, MD, Tomas Ondrus, MD, Lumir Koc, MD • 1 st Department of Internal Medicine, Faculty of Medicine, University Hospital Hradec Kralove, Charles University Prague, Czech Republic : Josef Stasek, MD, Ph. D, Ludek Haman, MD, Ph. D, Josef Bis, MD, Ph. D, Jiri Jäger, MD, Martina Tomkova, MD • Department of Cardiology, Cardiocenter, Hospital Podlesí a. s. , Trinec, Czech Republic : Marian Branny, MD, Ph. D, Jan Chovancik, MD, Ph. D, Libor Sknouril, MD, Ph. D • Department of Cardiology, Krajská zdravotni a. s. , Masaryk hospital and UJEP, Usti nad Labem, Czech Republic : Pavel Cervinka, MD, Ph. D, Jiri Holy, MD • Cardiocenter, 2 nd internal clinic – Cardiology and Angiology, Charles University, General Faculty Hospital, Prague Czech Republic : Tomas Kovarnik, MD, Ph. D, Stepan Havranek, MD, Ph. D, David Zemanek, MD, Ph. D • Department of Cardiology, University Hospital and Faculty of Medicine Pilsen, Czech Republic : Vlastimil Vancura, MD, Ph. D, Richard Rokyta, MD, Ph. D, Jan Lhotsky, MD • Cardiocenter, Institute of Clinical and Experimental Medicine, Prague, Czech Republic : Petr Peichl, MD, Ph. D, Bronislav Janek, MD, Prodrag Stojadinovic, MD • Statistics and IT, Masaryk University, Institute of Biostatistics and Analyses, Czech Republic: Dr. Jiri Jarkovsky, Ph. D, Sarka Haskova, Mgr, Martina Novackova, Mgr. , Klara Benesova, Mgr • DATA SAFETY AND MONTIORING BOARD: Jaromir Hradec, MD, CSc, Frantisek Tousek, MD, Rostislav Polasek, MD