NCDR Left Atrial Appendage Occlusion LAAO Registry Current











- Slides: 20

NCDR Left Atrial Appendage Occlusion (LAAO) Registry: Current Status and Opportunities David Slotwiner, MD, FACC, FHRS NCDR LAAO Registry Steering Committee Member

David Slotwiner, MD, FACC, FHRS I have no relevant financial relationships

Overview • • • Rationale for a Registry Multi-stakeholder Collaboration Registry Development Process Challenges Progress to Date

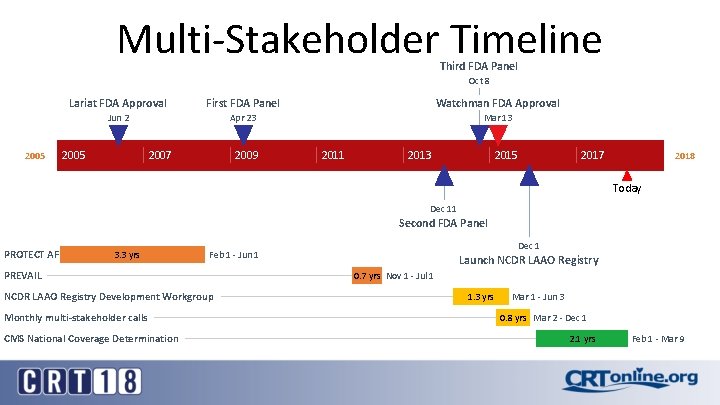
Multi-Stakeholder Timeline Third FDA Panel Oct 8 2005 Lariat FDA Approval First FDA Panel Watchman FDA Approval Jun 2 Apr 23 Mar 13 2005 2007 2009 2011 2013 2015 2017 2018 Today Dec 11 Second FDA Panel PROTECT AF 3. 3 yrs PREVAIL NCDR LAAO Registry Development Workgroup Monthly multi-stakeholder calls CMS National Coverage Determination Dec 1 Feb 1 - Jun 1 Launch NCDR LAAO Registry 0. 7 yrs Nov 1 - Jul 1 1. 3 yrs Mar 1 - Jun 3 0. 8 yrs Mar 2 - Dec 1 2. 1 yrs Feb 1 - Mar 9

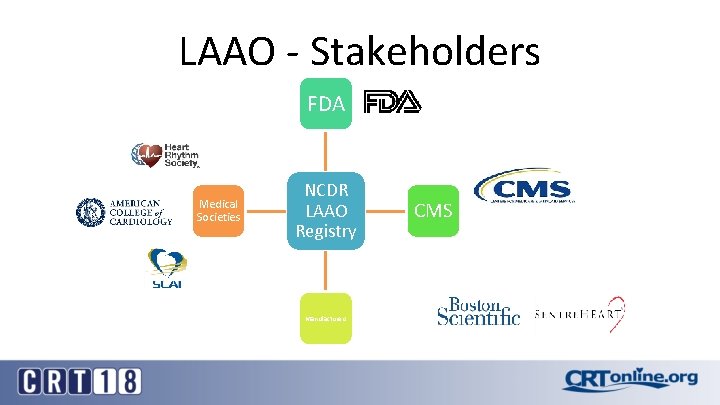
LAAO - Stakeholders FDA Medical Societies NCDR LAAO Registry Manufacturers CMS

2012 FDA: National Medical Device Postmarket Surveillance System (MDS) Goal: • Use real-world data • Quickly identify poorly performing devices • Generate data to support – premarket clearance of new devices and – new uses for currently marketed devices. 5 step plan released 2/23/15: • Create multi-stakeholder planning board • Establish Universal Device Identifier (UDI) • Promote development of national and international registries for selected products • Modernize adverse reporting system • Develop and use new methods for evidence generation, synthesis and approval

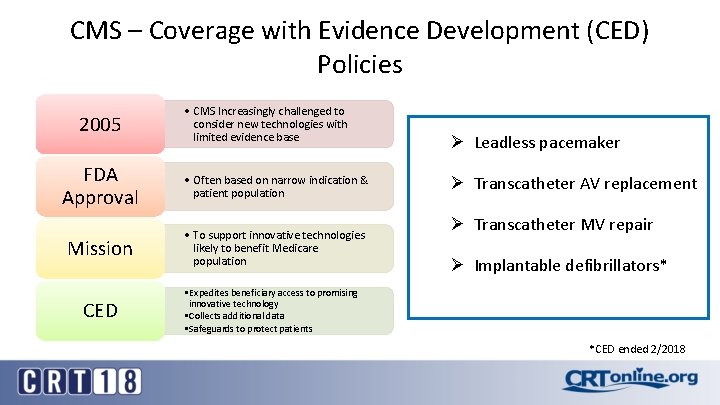
CMS – Coverage with Evidence Development (CED) Policies 2005 • CMS Increasingly challenged to consider new technologies with limited evidence base FDA Approval • Often based on narrow indication & patient population Mission • To support innovative technologies likely to benefit Medicare population CED Ø Leadless pacemaker Ø Transcatheter AV replacement Ø Transcatheter MV repair Ø Implantable defibrillators* • Expedites beneficiary access to promising innovative technology • Collects additional data • Safeguards to protect patients *CED ended 2/2018

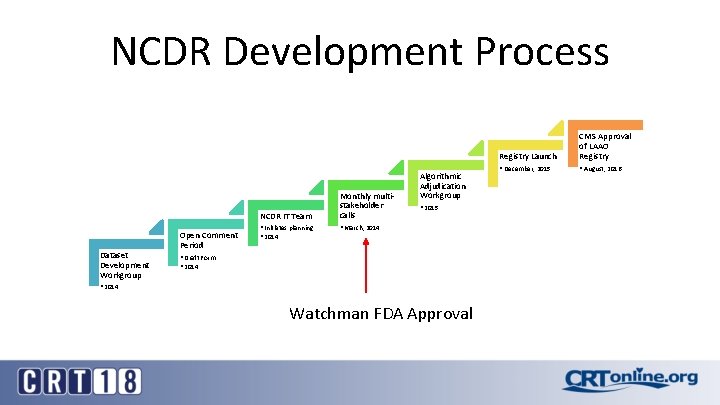
NCDR Development Process NCDR IT Team Dataset Development Workgroup Open Comment Period • Initiates planning • 2014 Monthly multistakeholder calls Algorithmic Adjudication Workgroup • 2015 • March, 2014 • Draft Form • 2014 Watchman FDA Approval Registry Launch CMS Approval of LAAO Registry • December, 2015 • August, 2016

Challenge: Outcomes Ascertainment Clinical Trials vs. Registries

Clinical Trials: Formal Adjudication of Outcomes Clinical Endpoints Committee (CEC): • Collect Data: – Hospital/prehospital records – Proxy interviews – Death certificates • Standardized protocols • Centralized endpoint adjudication

Clinical Trials: Formal Adjudication of Outcomes Clinical Endpoints Committee (CEC): • Collect Data: – Hospital/prehospital records but Rigorous, – Proxy interviews expensive. – Death certificates • Standardized protocols • Centralized endpoint adjudication

Quality-Focused Registries: • Site-level coding of endpoints – Typically without formal adjudication • Simple to capture on data collection forms • Relatively little staff time (compared to RCTs) • Less expensive

Quality-Focused Registries: • Site-level coding of endpoints – Typically without formal adjudication expensive, but forms • Simple to. Less capture on data collection potential for(compared bias • Relatively little staff time to RCTs) • Less expensive

LAAO Registry: Algorithmic Adjudication • Hybrid approach: structured capture of key data elements that comprise an endpoint definition • Targeted review of selected endpoints where algorithm proves insufficient • Sustainable: – Less expensive, – Employs standardized/centralized approaches.

LAAO Registry: Algorithmic Adjudication • Hybrid approach: structured capture of key data elements that comprise an endpoint definition • Targeted. Rigor review approaching of selected endpoints CECwhere algorithm proves insufficient adjudication, but with cost • Sustainable: sustainability of a registry – Less expensive, – Employs standardized/centralized approaches.

NCDR LAAO Registry Algorithmic Adjudication: – Neurological Events – Systemic Embolism

Enrollment

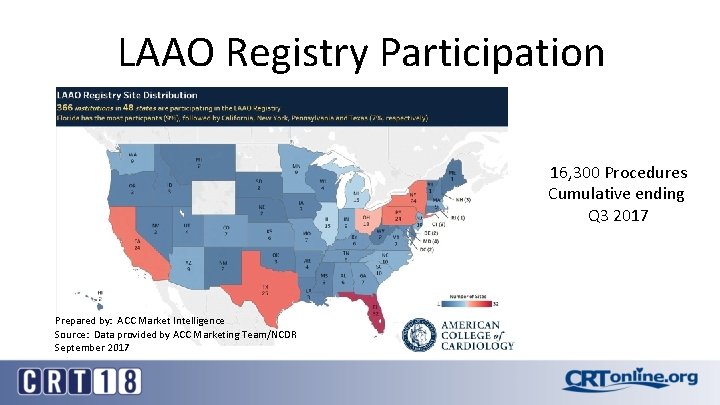
LAAO Registry Participation 16, 300 Procedures Cumulative ending Q 3 2017 Prepared by: ACC Market Intelligence Source: Data provided by ACC Marketing Team/NCDR September 2017

NCDR LAAO Upcoming Publications First Publication Descriptive information • Patient characteristics • Procedure characteristics Second Publication Outcomes Algorithmic adjudication • Describe the process • Report outcome of study comparing it with formal end-point adjudication

Conclusion NCDR LAAO Registry: Innovative Technologies: many stakeholders & many purposes Registries bridge FDA approval and real world use FDA Health Care Systems Medical Societies CMS NCDR LAAO Registry Manufacturers