WATCHMANTM Left Atrial Appendage Closure Device Clinical Data











- Slides: 50

WATCHMANTM Left Atrial Appendage Closure Device Clinical Data Overview SH 286002 AD MAY 2017

Agenda • Need for a Device-Based Alternative for Stroke Risk Reduction • WATCHMANTM Clinical Data – Clinical study overview – Efficacy – Stroke risk reduction – Efficacy – Bleeding reduction – Procedural Success and Safety – Future Patient Populations SH 286002 AD MAY 2017

Need for a Device-Based Alternative for Stroke Risk Reduction SH 286002 AD MAY 2017

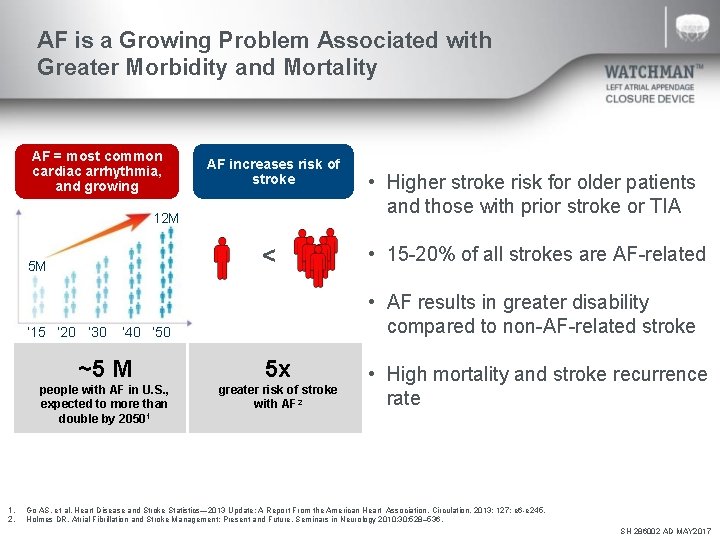
AF is a Growing Problem Associated with Greater Morbidity and Mortality AF = most common cardiac arrhythmia, and growing AF increases risk of stroke 12 M < 5 M • 15 -20% of all strokes are AF-related • AF results in greater disability compared to non-AF-related stroke ‘ 15 ‘ 20 ‘ 30 ’ 40 ‘ 50 1. 2. • Higher stroke risk for older patients and those with prior stroke or TIA ~5 M 5 x people with AF in U. S. , expected to more than double by 20501 greater risk of stroke with AF 2 • High mortality and stroke recurrence rate Go AS. et al, Heart Disease and Stroke Statistics— 2013 Update: A Report From the American Heart Association. Circulation. 2013; 127: e 6 -e 245. Holmes DR, Atrial Fibrillation and Stroke Management: Present and Future, Seminars in Neurology 2010; 30: 528– 536. SH 286002 AD MAY 2017

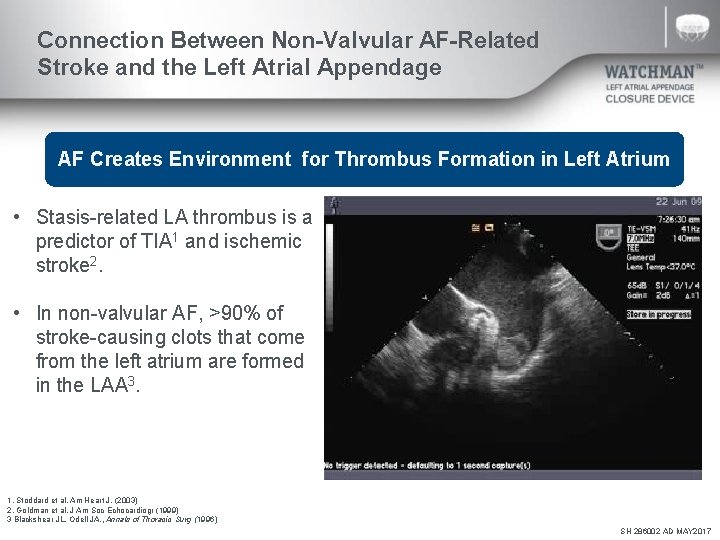
Connection Between Non-Valvular AF-Related Stroke and the Left Atrial Appendage AF Creates Environment for Thrombus Formation in Left Atrium • Stasis-related LA thrombus is a predictor of TIA 1 and ischemic stroke 2. • In non-valvular AF, >90% of stroke-causing clots that come from the left atrium are formed in the LAA 3. 1. Stoddard et al. Am Heart J. (2003) 2. Goldman et al. J Am Soc Echocardiogr (1999) 3 Blackshear JL. Odell JA. , Annals of Thoracic Surg (1996) SH 286002 AD MAY 2017

2014 ACC/AHA/HRS Treatment Guidelines to Prevent Thromboembolism in Patients with AF • Assess stroke risk with CHA 2 DS 2 -VASc score – Score 1: Annual stroke risk 1%, oral anticoagulants or aspirin may be considered – Score ≥ 2: Annual stroke risk 2%-15%, oral anticoagulants are recommended • Balance benefit vs. bleeding risk 2014 AHA/ACC/HRS Guideline for the Management of Patients with AF CHA 2 DS 2 VASc Score Recommendation 0 No anticoagulant 1 Aspirin (81 -325 mg daily) or warfarin (INR 2 -3) ≥ 2 Oral anticoagulants are recommended (warfarin (INR 2 -3), dabigatran, rivaroxaban or apixaban January, CT. et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. JACC. 2014; doi: 10. 1016/j. jacc. 2014. 03. 022 SH 286002 AD MAY 2017 N

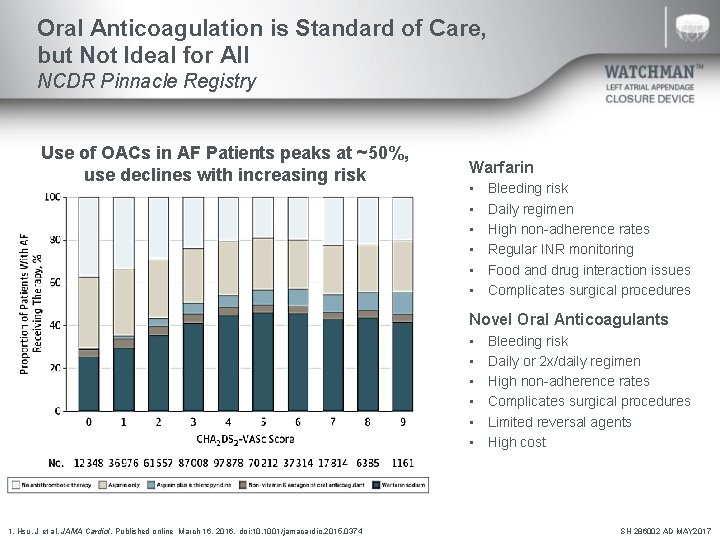
Oral Anticoagulation is Standard of Care, but Not Ideal for All NCDR Pinnacle Registry Use of OACs in AF Patients peaks at ~50%, use declines with increasing risk Warfarin • • • Bleeding risk Daily regimen High non-adherence rates Regular INR monitoring Food and drug interaction issues Complicates surgical procedures Novel Oral Anticoagulants • • • 1. Hsu, J et al. JAMA Cardiol. Published online March 16, 2016. doi: 10. 1001/jamacardio. 2015. 0374 Bleeding risk Daily or 2 x/daily regimen High non-adherence rates Complicates surgical procedures Limited reversal agents High cost SH 286002 AD MAY 2017

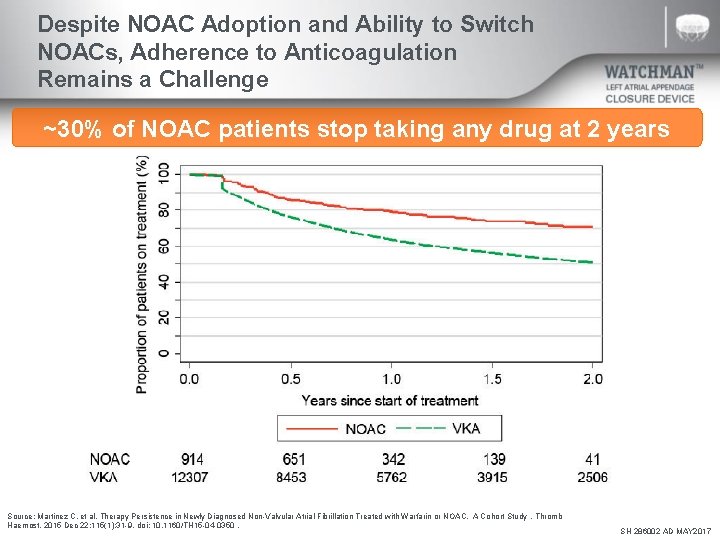
Despite NOAC Adoption and Ability to Switch NOACs, Adherence to Anticoagulation Remains a Challenge ~30% of NOAC patients stop taking any drug at 2 years Source: Martinez C, et al. Therapy Persistence in Newly Diagnosed Non-Valvular Atrial Fibrillation Treated with Warfarin or NOAC. A Cohort Study. Thromb Haemost. 2015 Dec 22; 115(1): 31 -9. doi: 10. 1160/TH 15 -04 -0350. SH 286002 AD MAY 2017

Introducing the WATCHMAN™ LAAC Device Indications for Use The WATCHMAN Device is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with nonvalvular atrial fibrillation who: – Are at increased risk for stroke and systemic embolism based on CHADS 2 or CHA 2 DS 2 -VASc scores and are recommended for anticoagulation therapy; – Are deemed by their physicians to be suitable for warfarin; and – Have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin SH 286002 AD MAY 2017

WATCHMAN Clinical Data Clinical Study Overview SH 286002 AD MAY 2017

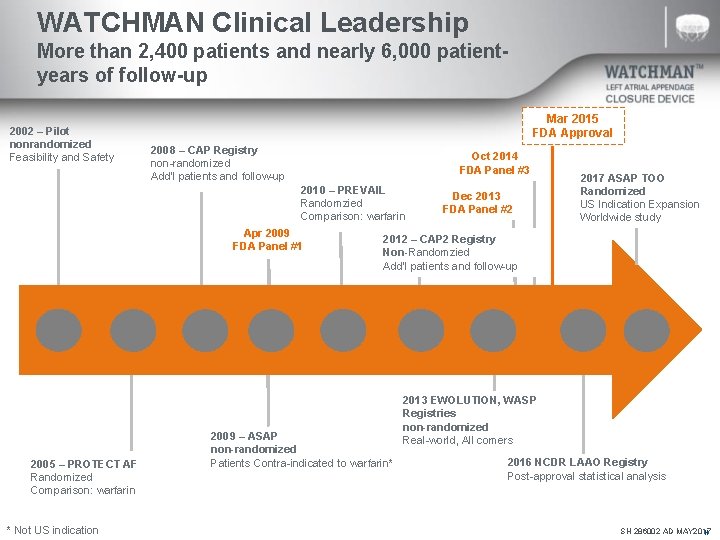
WATCHMAN Clinical Leadership More than 2, 400 patients and nearly 6, 000 patientyears of follow-up 2002 – Pilot nonrandomized Feasibility and Safety Mar 2015 FDA Approval 2008 – CAP Registry non-randomized Add’l patients and follow-up Oct 2014 FDA Panel #3 2010 – PREVAIL Randomzied Comparison: warfarin Apr 2009 FDA Panel #1 2005 – PROTECT AF Randomized Comparison: warfarin * Not US indication Dec 2013 FDA Panel #2 2017 ASAP TOO Randomized US Indication Expansion Worldwide study 2012 – CAP 2 Registry Non-Randomzied Add’l patients and follow-up 2009 – ASAP non-randomized Patients Contra-indicated to warfarin* 2013 EWOLUTION, WASP Registries non-randomized Real-world, All comers 2016 NCDR LAAO Registry Post-approval statistical analysis SH 286002 AD MAY 2017 N

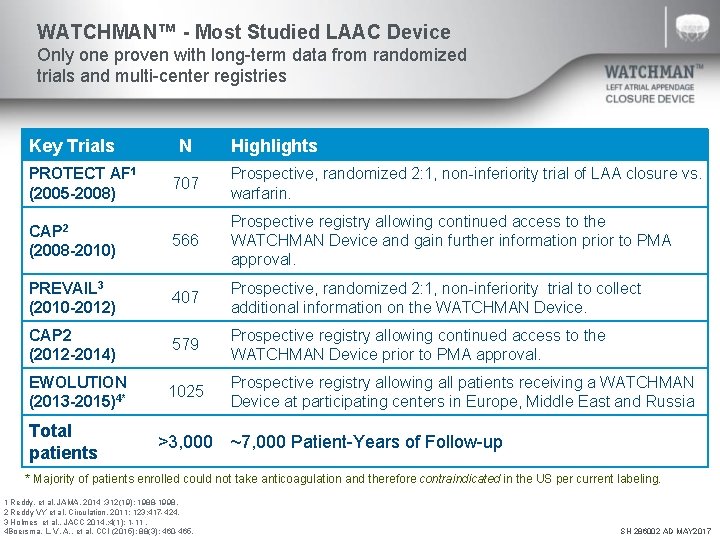
WATCHMAN™ - Most Studied LAAC Device Only one proven with long-term data from randomized trials and multi-center registries Key Trials PROTECT AF 1 (2005 -2008) N Highlights 707 Prospective, randomized 2: 1, non-inferiority trial of LAA closure vs. warfarin. CAP 2 (2008 -2010) 566 Prospective registry allowing continued access to the WATCHMAN Device and gain further information prior to PMA approval. PREVAIL 3 (2010 -2012) 407 Prospective, randomized 2: 1, non-inferiority trial to collect additional information on the WATCHMAN Device. CAP 2 (2012 -2014) 579 Prospective registry allowing continued access to the WATCHMAN Device prior to PMA approval. EWOLUTION (2013 -2015)4* 1025 Prospective registry allowing all patients receiving a WATCHMAN Device at participating centers in Europe, Middle East and Russia Total patients >3, 000 ~7, 000 Patient-Years of Follow-up * Majority of patients enrolled could not take anticoagulation and therefore contraindicated in the US per current labeling. 1 Reddy, et al. JAMA. 2014 ; 312(19): 1988 -1998. 2 Reddy VY et al. Circulation. 2011; 123: 417 -424. 3 Holmes et al. , JACC 2014, ; 4(1): 1 -11. 4 Boersma, L. V. A. , et al. CCI (2015); 88(3): 460 -465. SH 286002 AD MAY 2017

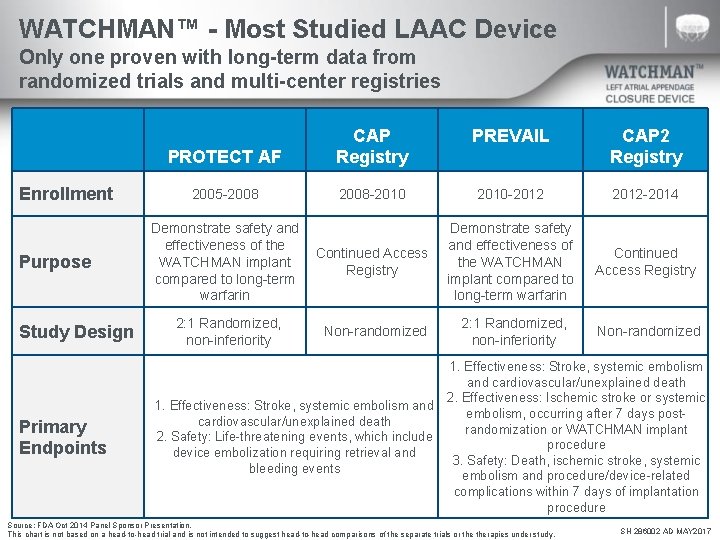
WATCHMAN™ - Most Studied LAAC Device Only one proven with long-term data from randomized trials and multi-center registries Enrollment Purpose Study Design Primary Endpoints PREVAIL PROTECT AF CAP Registry CAP 2 Registry 2005 -2008 -2010 -2012 -2014 Demonstrate safety and effectiveness of the WATCHMAN implant compared to long-term warfarin Continued Access Registry 2: 1 Randomized, non-inferiority Non-randomized Demonstrate safety and effectiveness of the Continued Access WATCHMAN implant Registry compared to long-term warfarin 2: 1 Randomized, non-inferiority Non-randomized 1. Effectiveness: Stroke, systemic embolism and cardiovascular/unexplained death 2. Effectiveness: Ischemic stroke or systemic 1. Effectiveness: Stroke, systemic embolism and embolism, occurring after 7 days postcardiovascular/unexplained death randomization or WATCHMAN implant 2. Safety: Life-threatening events, which include procedure device embolization requiring retrieval and 3. Safety: Death, ischemic stroke, systemic bleeding events embolism and procedure/device-related complications within 7 days of implantation procedure Source: FDA Oct 2014 Panel Sponsor Presentation. This chart is not based on a head-to-head trial and is not intended to suggest head-to-head comparisons of the separate trials or therapies under study. SH 286002 AD MAY 2017

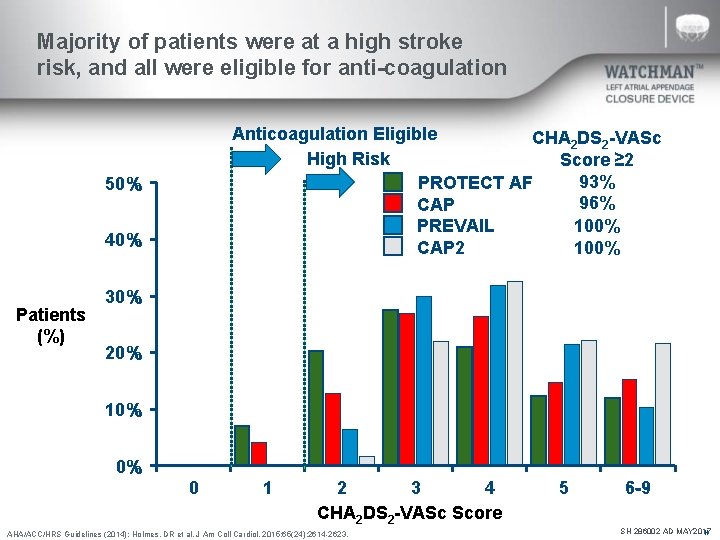
Majority of patients were at a high stroke risk, and all were eligible for anti-coagulation Anticoagulation Eligible CHA 2 DS 2 -VASc High Risk Score ≥ 2 93% PROTECT AF 96% CAP 100% PREVAIL 100% CAP 2 50% 40% Patients (%) 30% 20% 10% 0% 0 1 2 3 4 CHA 2 DS 2 -VASc Score AHA/ACC/HRS Guidelines (2014); Holmes, DR et al. J Am Coll Cardiol. 2015; 65(24): 2614 -2623. 5 6 -9 SH 286002 AD MAY 2017 N

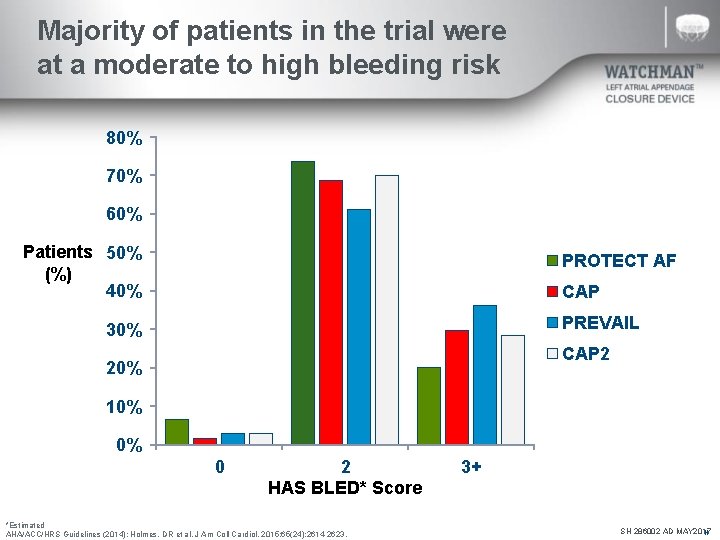
Majority of patients in the trial were at a moderate to high bleeding risk 80% 70% 60% Patients 50% (%) 40% PROTECT AF CAP PREVAIL 30% CAP 2 20% 10% 0% 0 2 HAS BLED* Score *Estimated AHA/ACC/HRS Guidelines (2014); Holmes, DR et al. J Am Coll Cardiol. 2015; 65(24): 2614 -2623. 3+ SH 286002 AD MAY 2017 N

WATCHMAN Clinical Data Efficacy – Stroke Risk Reduction SH 286002 AD MAY 2017

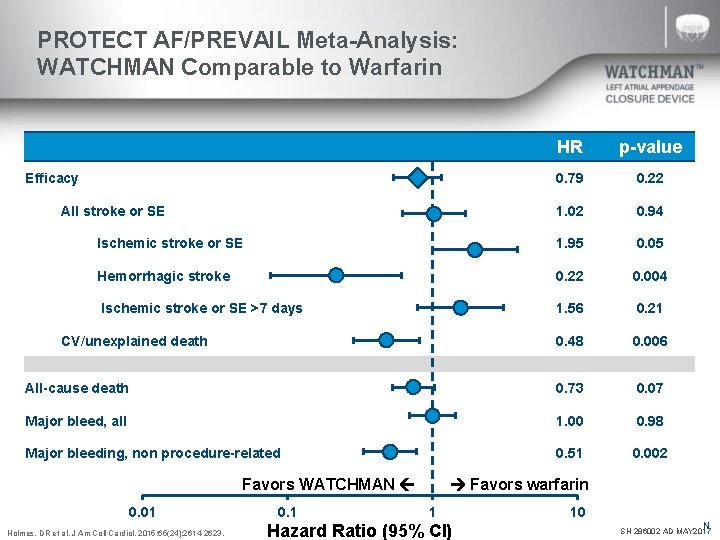
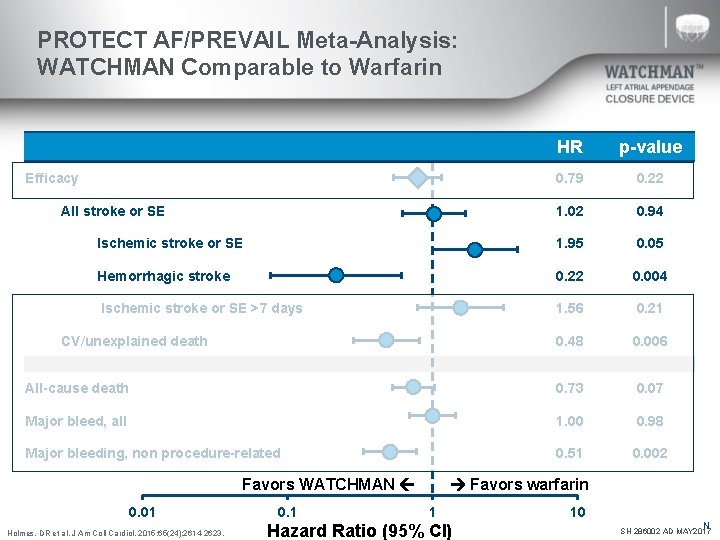
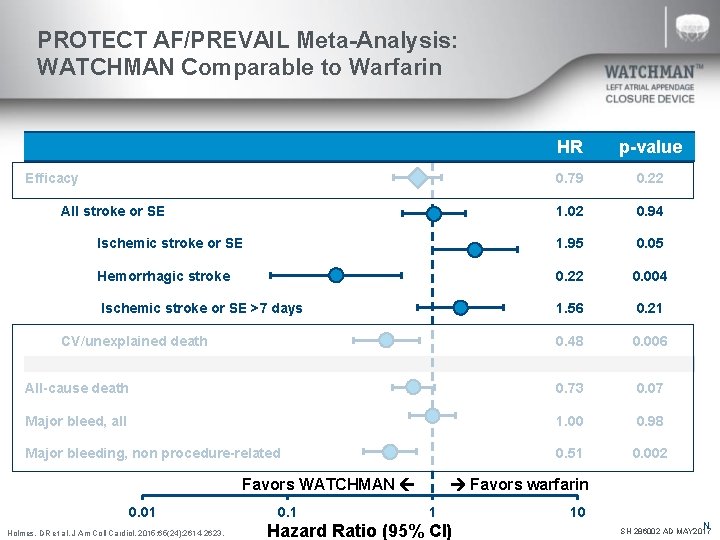
PROTECT AF/PREVAIL Meta-Analysis: WATCHMAN Comparable to Warfarin HR p-value 0. 79 0. 22 1. 02 0. 94 Ischemic stroke or SE 1. 95 0. 05 Hemorrhagic stroke 0. 22 0. 004 Ischemic stroke or SE >7 days 1. 56 0. 21 0. 48 0. 006 All-cause death 0. 73 0. 07 Major bleed, all 1. 00 0. 98 Major bleeding, non procedure-related 0. 51 0. 002 Efficacy All stroke or SE CV/unexplained death Favors WATCHMAN 0. 01 Holmes, DR et al. J Am Coll Cardiol. 2015; 65(24): 2614 -2623. 0. 1 Favors warfarin 1 Hazard Ratio (95% CI) 10 N SH 286002 AD MAY 2017

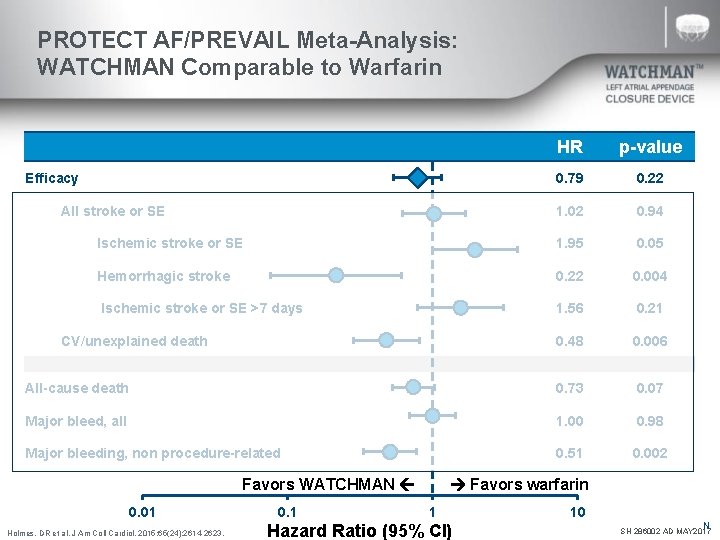
PROTECT AF/PREVAIL Meta-Analysis: WATCHMAN Comparable to Warfarin HR p-value 0. 79 0. 22 1. 02 0. 94 Ischemic stroke or SE 1. 95 0. 05 Hemorrhagic stroke 0. 22 0. 004 Ischemic stroke or SE >7 days 1. 56 0. 21 0. 48 0. 006 All-cause death 0. 73 0. 07 Major bleed, all 1. 00 0. 98 Major bleeding, non procedure-related 0. 51 0. 002 Efficacy All stroke or SE CV/unexplained death Favors WATCHMAN 0. 01 Holmes, DR et al. J Am Coll Cardiol. 2015; 65(24): 2614 -2623. 0. 1 Favors warfarin 1 Hazard Ratio (95% CI) 10 N SH 286002 AD MAY 2017

PROTECT AF/PREVAIL Meta-Analysis: WATCHMAN Comparable to Warfarin HR p-value 0. 79 0. 22 1. 02 0. 94 Ischemic stroke or SE 1. 95 0. 05 Hemorrhagic stroke 0. 22 0. 004 Ischemic stroke or SE >7 days 1. 56 0. 21 0. 48 0. 006 All-cause death 0. 73 0. 07 Major bleed, all 1. 00 0. 98 Major bleeding, non procedure-related 0. 51 0. 002 Efficacy All stroke or SE CV/unexplained death Favors WATCHMAN 0. 01 Holmes, DR et al. J Am Coll Cardiol. 2015; 65(24): 2614 -2623. 0. 1 Favors warfarin 1 Hazard Ratio (95% CI) 10 N SH 286002 AD MAY 2017

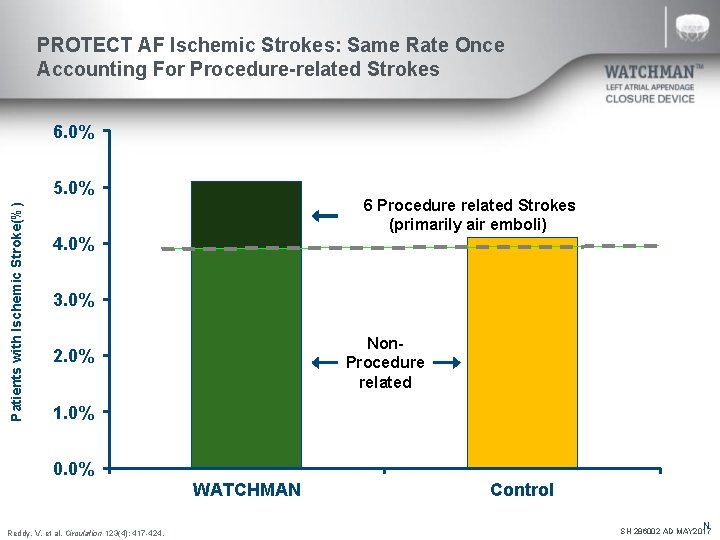
PROTECT AF Ischemic Strokes: Same Rate Once Accounting For Procedure-related Strokes 6. 0% Patients with Ischemic Stroke(%) 5. 0% 6 Procedure related Strokes (primarily air emboli) 4. 0% 3. 0% Non- Procedure related 2. 0% 1. 0% 0. 0% WATCHMAN Reddy, V. et al. Circulation 123(4): 417 -424. Control N SH 286002 AD MAY 2017

PROTECT AF/PREVAIL Meta-Analysis: WATCHMAN Comparable to Warfarin HR p-value 0. 79 0. 22 1. 02 0. 94 Ischemic stroke or SE 1. 95 0. 05 Hemorrhagic stroke 0. 22 0. 004 Ischemic stroke or SE >7 days 1. 56 0. 21 0. 48 0. 006 All-cause death 0. 73 0. 07 Major bleed, all 1. 00 0. 98 Major bleeding, non procedure-related 0. 51 0. 002 Efficacy All stroke or SE CV/unexplained death Favors WATCHMAN 0. 01 Holmes, DR et al. J Am Coll Cardiol. 2015; 65(24): 2614 -2623. 0. 1 Favors warfarin 1 Hazard Ratio (95% CI) 10 N SH 286002 AD MAY 2017

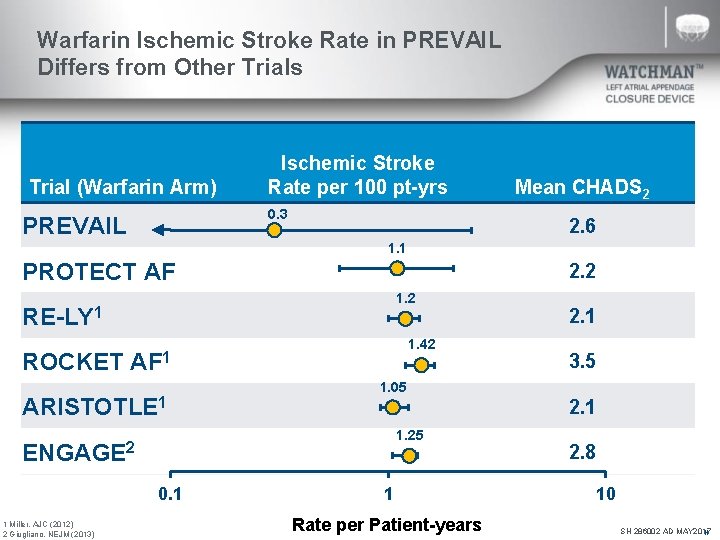
Warfarin Ischemic Stroke Rate in PREVAIL Differs from Other Trials Trial (Warfarin Arm) Ischemic Stroke Rate per 100 pt-yrs 0. 3 PREVAIL Mean CHADS 2 2. 6 1. 1 PROTECT AF 2. 2 1. 2 RE-LY 1 1. 42 ROCKET AF 1 ARISTOTLE 1 1 Miller. AJC (2012) 2 Giugliano. NEJM (2013) 3. 5 1. 05 2. 1 1. 25 ENGAGE 2 0. 1 2. 1 1 Rate per Patient-years 2. 8 10 SH 286002 AD MAY 2017 N

- FDA Panel (Oct. 2014) SH 286002 AD MAY 2017

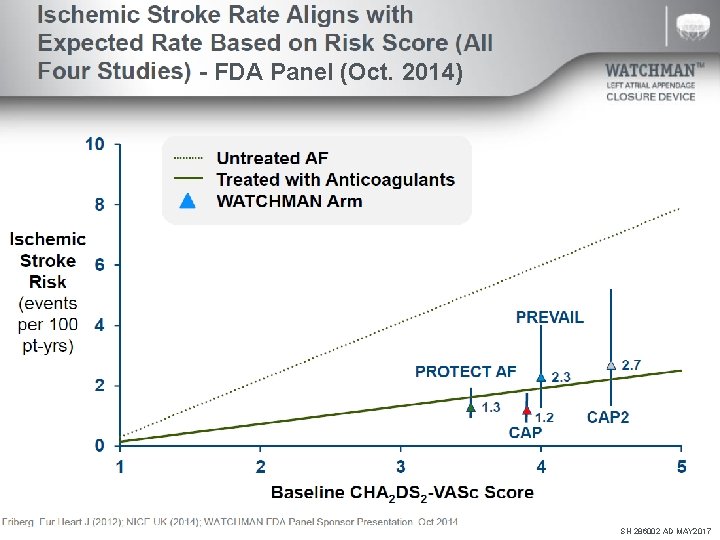
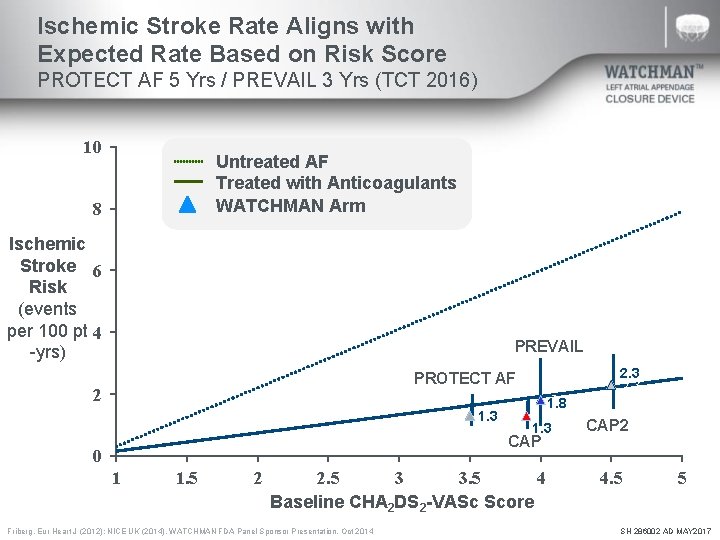
Ischemic Stroke Rate Aligns with Expected Rate Based on Risk Score PROTECT AF 5 Yrs / PREVAIL 3 Yrs (TCT 2016) 10 Untreated AF Treated with Anticoagulants WATCHMAN Arm 8 Ischemic Stroke 6 Risk (events per 100 pt 4 -yrs) PREVAIL PROTECT AF 2 1. 3 1. 8 1. 3 CAP 0 1 1. 5 2 2. 5 3 3. 5 4 Baseline CHA 2 DS 2 -VASc Score Friberg. Eur Heart J (2012); NICE UK (2014). WATCHMAN FDA Panel Sponsor Presentation. Oct 2014 2. 3 CAP 2 4. 5 5 SH 286002 AD MAY 2017

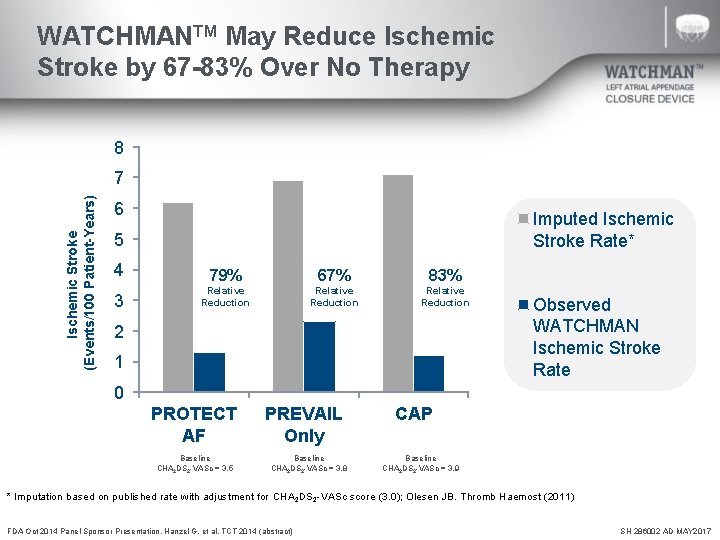
WATCHMANTM May Reduce Ischemic Stroke by 67 -83% Over No Therapy 8 Ischemic Stroke (Events/100 Patient-Years) 7 6 Imputed Ischemic Stroke Rate* 5 4 79% 67% 83% 3 Relative Reduction 2 1 Observed WATCHMAN Ischemic Stroke Rate 0 PROTECT AF PREVAIL Only Baseline CHA 2 DS 2 -VASc = 3. 5 Baseline CHA 2 DS 2 -VASc = 3. 8 CAP Baseline CHA 2 DS 2 -VASc = 3. 9 * Imputation based on published rate with adjustment for CHA 2 DS 2 -VASc score (3. 0); Olesen JB. Thromb Haemost (2011) FDA Oct 2014 Panel Sponsor Presentation. Hanzel G, et al. TCT 2014 (abstract) SH 286002 AD MAY 2017

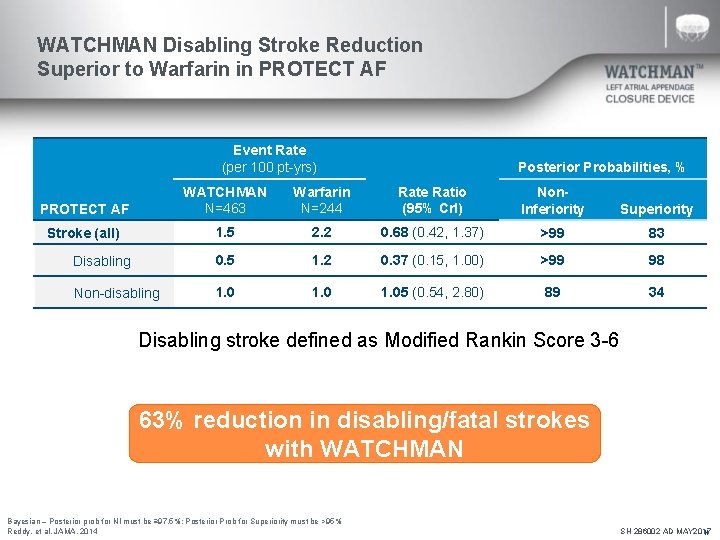
WATCHMAN Disabling Stroke Reduction Superior to Warfarin in PROTECT AF Event Rate (per 100 pt-yrs) Posterior Probabilities, % PROTECT AF WATCHMAN N=463 Warfarin N=244 Rate Ratio (95% Cr. I) Non. Inferiority Superiority Stroke (all) 1. 5 2. 2 0. 68 (0. 42, 1. 37) >99 83 0. 5 1. 2 0. 37 (0. 15, 1. 00) >99 98 1. 05 (0. 54, 2. 80) 89 34 Disabling Non-disabling Disabling stroke defined as Modified Rankin Score 3 -6 63% reduction in disabling/fatal strokes with WATCHMAN Bayesian – Posterior prob for NI must be ≥ 97. 5%; Posterior Prob for Superiority must be >95% Reddy, et al. JAMA. 2014 SH 286002 AD MAY 2017 N

WATCHMAN Clinical Data Efficacy – Bleeding Reduction SH 286002 AD MAY 2017

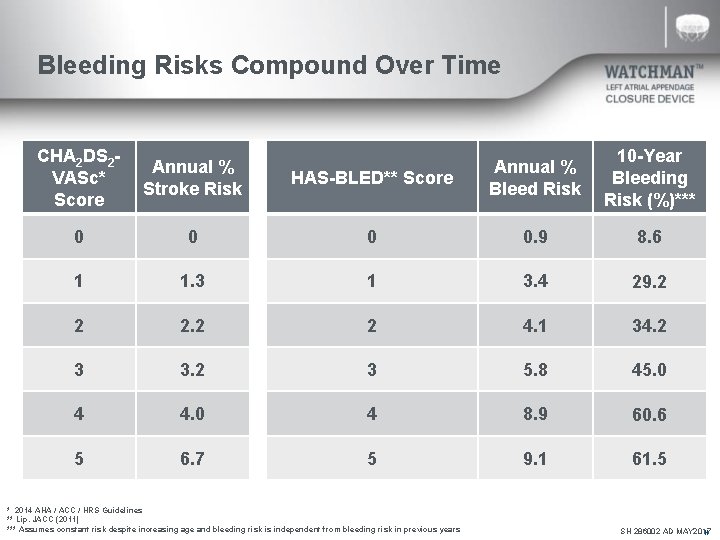
Bleeding Risks Compound Over Time CHA 2 DS 2 VASc* Score Annual % Stroke Risk HAS-BLED** Score Annual % Bleed Risk 10 -Year Bleeding Risk (%)*** 0 0. 9 8. 6 1 1. 3 1 3. 4 29. 2 2 2 4. 1 34. 2 3 3. 2 3 5. 8 45. 0 4 4. 0 4 8. 9 60. 6 5 6. 7 5 9. 1 61. 5 * 2014 AHA / ACC / HRS Guidelines ** Lip. JACC (2011) *** Assumes constant risk despite increasing age and bleeding risk is independent from bleeding risk in previous years SH 286002 AD MAY 2017 N

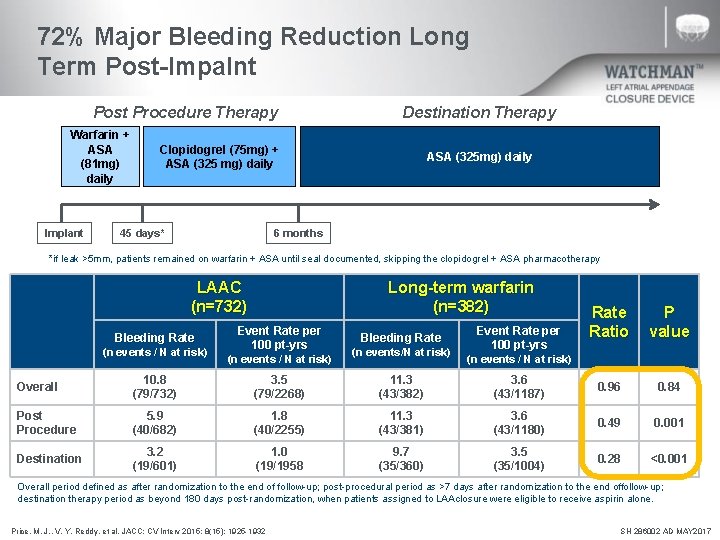
72% Major Bleeding Reduction Long Term Post-Impalnt Post Procedure Therapy Warfarin + ASA (81 mg) daily Implant Destination Therapy Clopidogrel (75 mg) + ASA (325 mg) daily 45 days* ASA (325 mg) daily 6 months *if leak >5 mm, patients remained on warfarin + ASA until seal documented, skipping the clopidogrel + ASA pharmacotherapy LAAC (n=732) Bleeding Rate (n events / N at risk) Long-term warfarin (n=382) Event Rate per 100 pt-yrs (n events / N at risk) Bleeding Rate (n events/N at risk) Event Rate per 100 pt-yrs Rate Ratio P value (n events / N at risk) Overall 10. 8 (79/732) 3. 5 (79/2268) 11. 3 (43/382) 3. 6 (43/1187) 0. 96 0. 84 Post Procedure 5. 9 (40/682) 1. 8 (40/2255) 11. 3 (43/381) 3. 6 (43/1180) 0. 49 0. 001 Destination 3. 2 (19/601) 1. 0 (19/1958 9. 7 (35/360) 3. 5 (35/1004) 0. 28 <0. 001 Overall period defined as after randomization to the end of follow-up; post-procedural period as >7 days after randomization to the end of follow-up; destination therapy period as beyond 180 days post-randomization, when patients assigned to LAA closure were eligible to receive aspirin alone. Price, M. J. , V. Y. Reddy, et al. JACC: CV Interv 2015; 8(15): 1925 -1932 SH 286002 AD MAY 2017

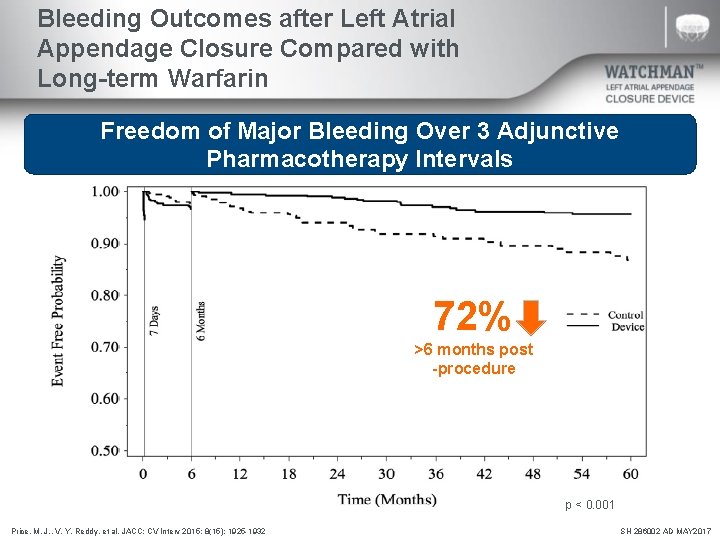
Bleeding Outcomes after Left Atrial Appendage Closure Compared with Long-term Warfarin Freedom of Major Bleeding Over 3 Adjunctive Pharmacotherapy Intervals 72% >6 months post -procedure p < 0. 001 Price, M. J. , V. Y. Reddy, et al. JACC: CV Interv 2015; 8(15): 1925 -1932 SH 286002 AD MAY 2017

WATCHMAN Clinical Data Procedural Success and Safety SH 286002 AD MAY 2017

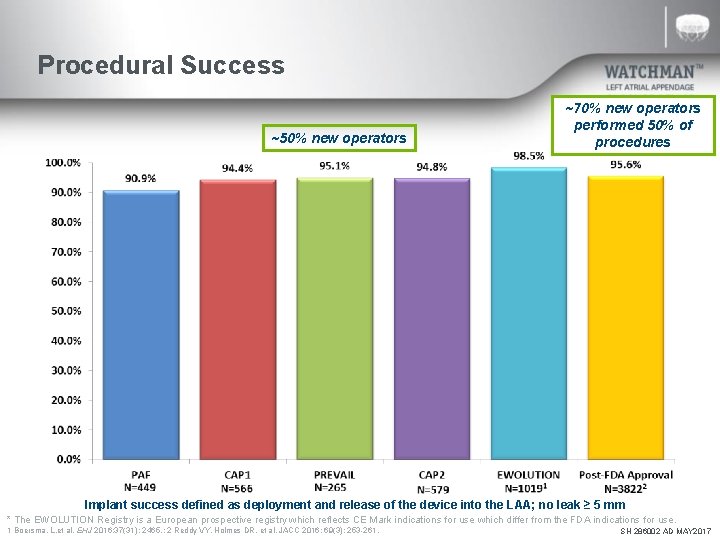
Procedural Success ~50% new operators ~70% new operators performed 50% of procedures Implant success defined as deployment and release of the device into the LAA; no leak ≥ 5 mm * The EWOLUTION Registry is a European prospective registry which reflects CE Mark indications for use which differ from the FDA indications for use. 1 Boersma, L. et al. EHJ 2016; 37(31): 2465. ; 2 Reddy VY, Holmes DR, et al. JACC 2016; 69(3): 253 -261. SH 286002 AD MAY 2017

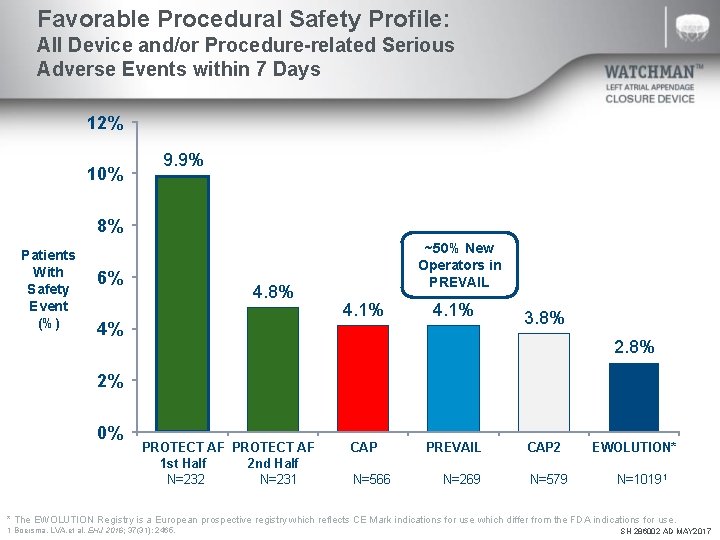
Favorable Procedural Safety Profile: All Device and/or Procedure-related Serious Adverse Events within 7 Days 12% 10% 9. 9% 8% Patients With Safety Event (%) 6% 4. 8% 4% ~50% New Operators in PREVAIL 4. 1% 3. 8% 2% 0% PROTECT AF 1 st Half 2 nd Half N=232 N=231 CAP N=566 PREVAIL CAP 2 N=269 N=579 EWOLUTION* N=10191 * The EWOLUTION Registry is a European prospective registry which reflects CE Mark indications for use which differ from the FDA indications for use. 1 Boersma, LVA. et al. EHJ 2016; 37(31): 2465. SH 286002 AD MAY 2017

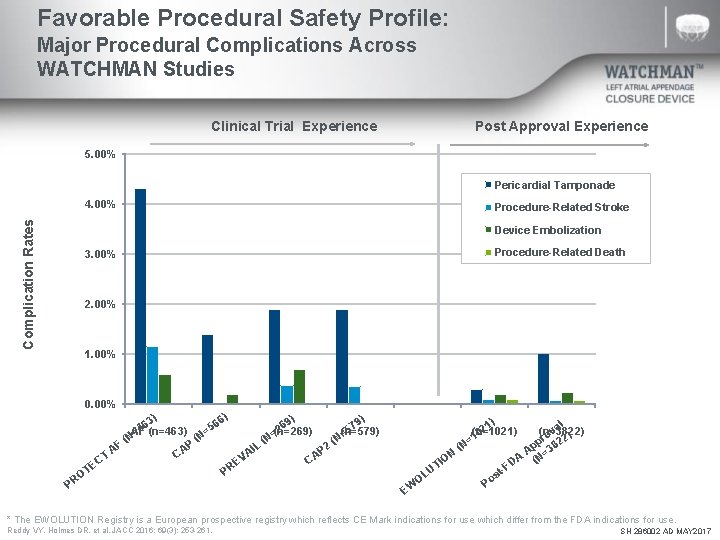
Favorable Procedural Safety Profile: Major Procedural Complications Across WATCHMAN Studies Clinical Trial Experience Post Approval Experience 5. 00% Pericardial Tamponade Complication Rates 4. 00% Procedure-Related Stroke Device Embolization 3. 00% Procedure-Related Death 2. 00% 1. 00% 0. 00% PROTECT CAP (n=566) PREVAIL CAP 2 ) ) 6) 3) 6 6 69 79 5 4 2 5 AF (n=463) (n=269) (n=579) = = (N (N P F 2 L I A CA AP T VA C C E E PR OT PR EWOLUTION US Cohort 1) al 2 (n=1021) (n=3822) v 0 ) o =1 pr 822 p ( N 3 A A (N= ON I D -F UT st o OL P EW * The EWOLUTION Registry is a European prospective registry which reflects CE Mark indications for use which differ from the FDA indications for use. Reddy VY, Holmes DR, et al. JACC 2016; 69(3): 253 -261. SH 286002 AD MAY 2017

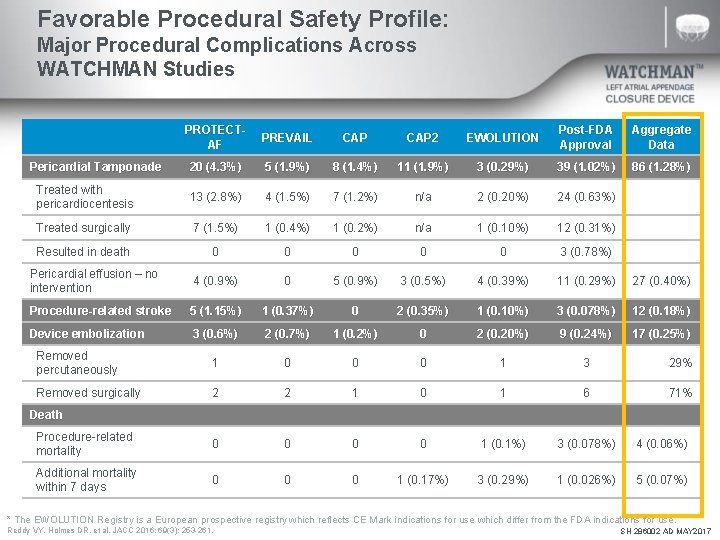
Favorable Procedural Safety Profile: Major Procedural Complications Across WATCHMAN Studies PROTECTAF PREVAIL CAP 2 EWOLUTION Post-FDA Approval Aggregate Data Pericardial Tamponade 20 (4. 3%) 5 (1. 9%) 8 (1. 4%) 11 (1. 9%) 3 (0. 29%) 39 (1. 02%) 86 (1. 28%) Treated with pericardiocentesis 13 (2. 8%) 4 (1. 5%) 7 (1. 2%) n/a 2 (0. 20%) 24 (0. 63%) Treated surgically 7 (1. 5%) 1 (0. 4%) 1 (0. 2%) n/a 1 (0. 10%) 12 (0. 31%) Resulted in death 0 0 0 3 (0. 78%) Pericardial effusion – no intervention 4 (0. 9%) 0 5 (0. 9%) 3 (0. 5%) 4 (0. 39%) 11 (0. 29%) 27 (0. 40%) Procedure-related stroke 5 (1. 15%) 1 (0. 37%) 0 2 (0. 35%) 1 (0. 10%) 3 (0. 078%) 12 (0. 18%) Device embolization 3 (0. 6%) 2 (0. 7%) 1 (0. 2%) 0 2 (0. 20%) 9 (0. 24%) 17 (0. 25%) Removed percutaneously 1 0 0 0 1 3 29% Removed surgically 2 2 1 0 1 6 71% Procedure-related mortality 0 0 1 (0. 1%) 3 (0. 078%) 4 (0. 06%) Additional mortality within 7 days 0 0 0 1 (0. 17%) 3 (0. 29%) 1 (0. 026%) 5 (0. 07%) Death * The EWOLUTION Registry is a European prospective registry which reflects CE Mark indications for use which differ from the FDA indications for use. Reddy VY, Holmes DR, et al. JACC 2016; 69(3): 253 -261. SH 286002 AD MAY 2017

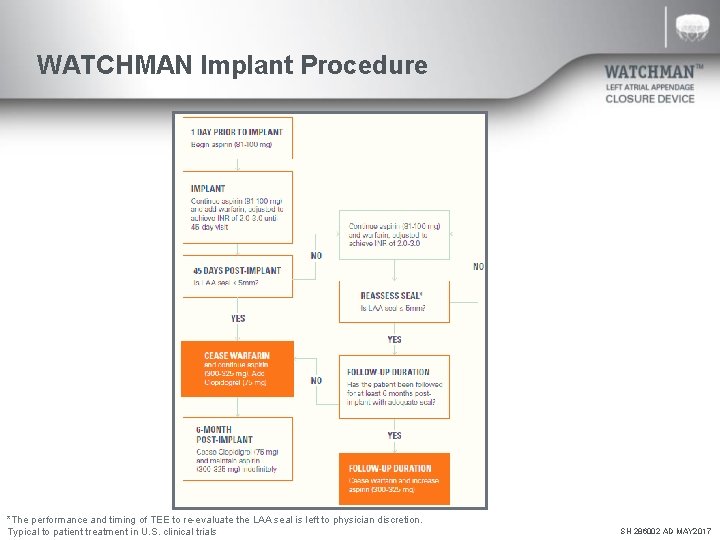
WATCHMAN Implant Procedure *The performance and timing of TEE to re-evaluate the LAA seal is left to physician discretion. Typical to patient treatment in U. S. clinical trials SH 286002 AD MAY 2017

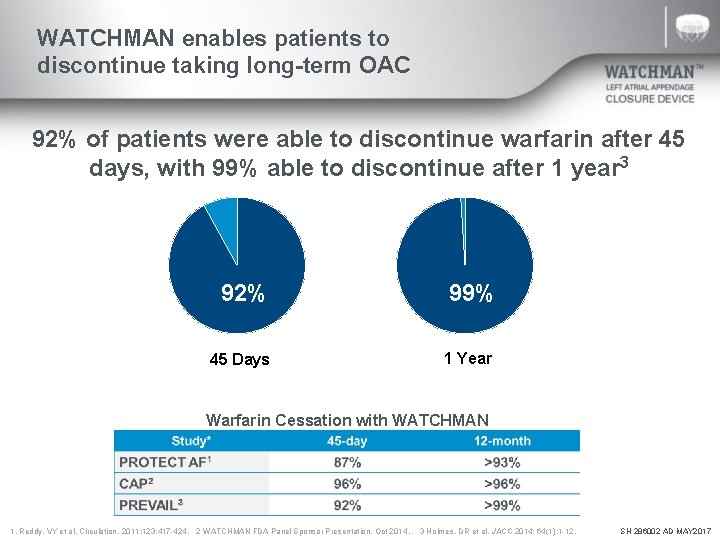
WATCHMAN enables patients to discontinue taking long-term OAC 92% of patients were able to discontinue warfarin after 45 days, with 99% able to discontinue after 1 year 3 92% 45 Days 92% 99% 1 Year Warfarin Cessation with WATCHMAN 1. Reddy, VY et al. Circulation. 2011; 123: 417 -424. 2 WATCHMAN FDA Panel Sponsor Presentation. Oct 2014. . 3 Holmes, DR et al. JACC 2014; 64(1): 1 -12. SH 286002 AD MAY 2017

WATCHMAN Clinical Data Future Patient Populations* * Data presented is in patients primarily contraindicated for LAAC with WATCHMAN in the United States. SH 286002 AD MAY 2017

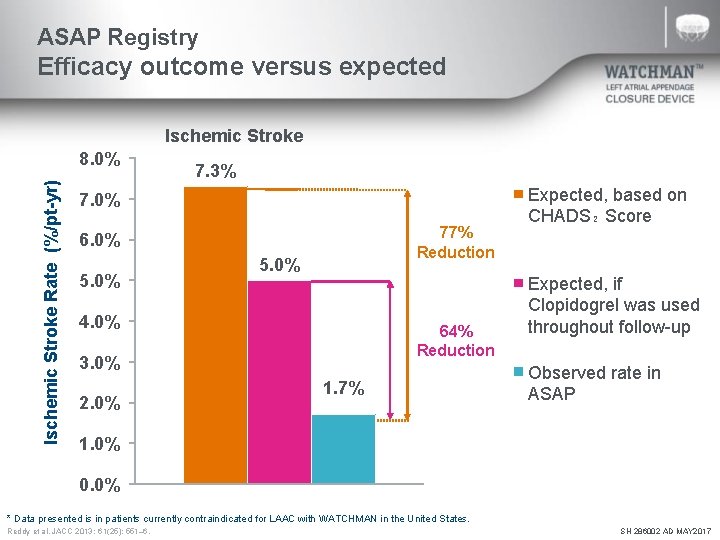
ASAP Registry Efficacy outcome versus expected Ischemic Stroke Rate (%/pt-yr) 8. 0% 7. 3% 7. 0% 77% Reduction 6. 0% 5. 0% 4. 0% 64% Reduction 3. 0% 2. 0% 1. 7% Expected, based on CHADS₂ Score Expected, if Clopidogrel was used throughout follow-up Observed rate in ASAP 1. 0% 0. 0% * Data presented is in patients currently contraindicated for LAAC with WATCHMAN in the United States. Reddy et al. JACC 2013; 61(25): 551– 6. SH 286002 AD MAY 2017

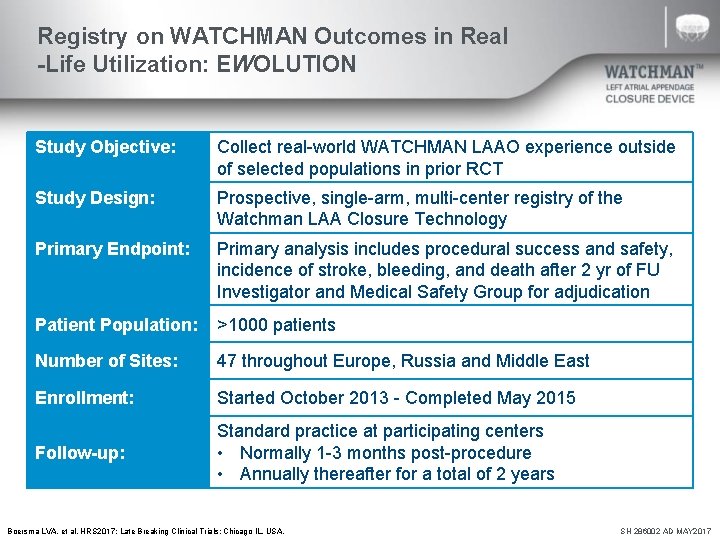
Registry on WATCHMAN Outcomes in Real -Life Utilization: EWOLUTION Study Objective: Collect real-world WATCHMAN LAAO experience outside of selected populations in prior RCT Study Design: Prospective, single-arm, multi-center registry of the Watchman LAA Closure Technology Primary Endpoint: Primary analysis includes procedural success and safety, incidence of stroke, bleeding, and death after 2 yr of FU Investigator and Medical Safety Group for adjudication Patient Population: >1000 patients Number of Sites: 47 throughout Europe, Russia and Middle East Enrollment: Started October 2013 - Completed May 2015 Follow-up: Standard practice at participating centers • Normally 1 -3 months post-procedure • Annually thereafter for a total of 2 years Boersma LVA, et al. HRS 2017; Late Breaking Clinical Trials: Chicago IL, USA. SH 286002 AD MAY 2017

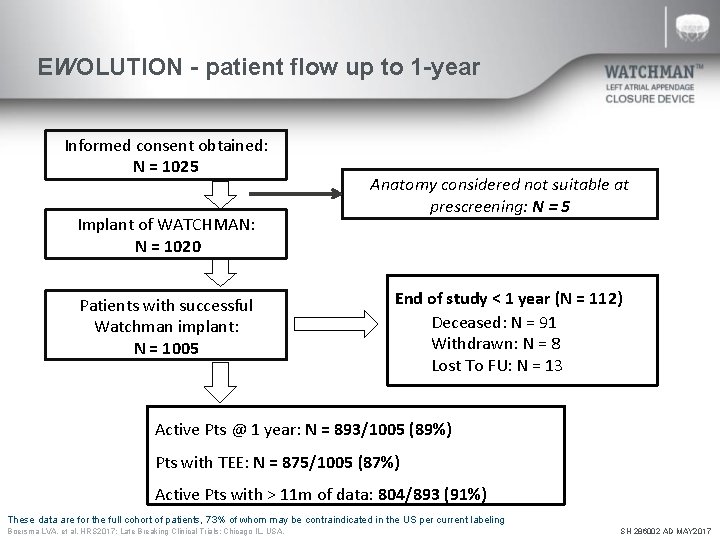
EWOLUTION - patient flow up to 1 -year Informed consent obtained: N = 1025 Implant of WATCHMAN: N = 1020 Patients with successful Watchman implant: N = 1005 Anatomy considered not suitable at prescreening: N = 5 End of study < 1 year (N = 112) Deceased: N = 91 Withdrawn: N = 8 Lost To FU: N = 13 Active Pts @ 1 year: N = 893/1005 (89%) Pts with TEE: N = 875/1005 (87%) Active Pts with > 11 m of data: 804/893 (91%) These data are for the full cohort of patients, 73% of whom may be contraindicated in the US per current labeling Boersma LVA, et al. HRS 2017; Late Breaking Clinical Trials: Chicago IL, USA. SH 286002 AD MAY 2017

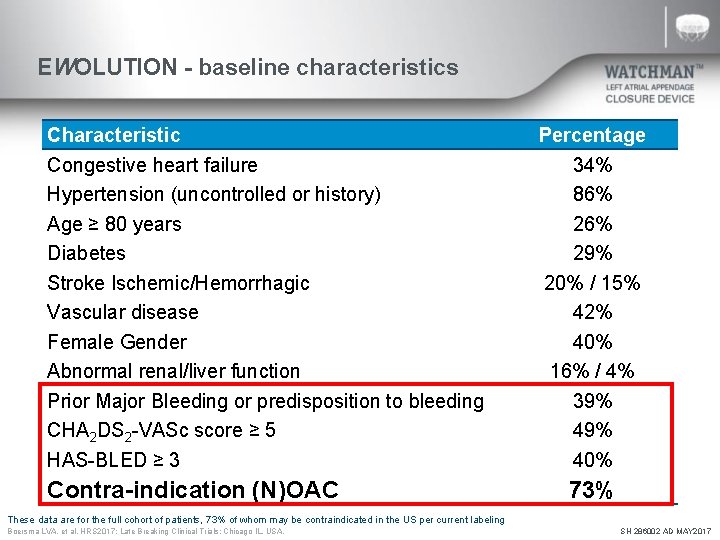
EWOLUTION - baseline characteristics Characteristic Congestive heart failure Hypertension (uncontrolled or history) Age ≥ 80 years Diabetes Stroke Ischemic/Hemorrhagic Vascular disease Female Gender Abnormal renal/liver function Prior Major Bleeding or predisposition to bleeding CHA 2 DS 2 -VASc score ≥ 5 HAS-BLED ≥ 3 Contra-indication (N)OAC Percentage 34% 86% 29% 20% / 15% 42% 40% 16% / 4% 39% 40% 73% These data are for the full cohort of patients, 73% of whom may be contraindicated in the US per current labeling Boersma LVA, et al. HRS 2017; Late Breaking Clinical Trials: Chicago IL, USA. SH 286002 AD MAY 2017

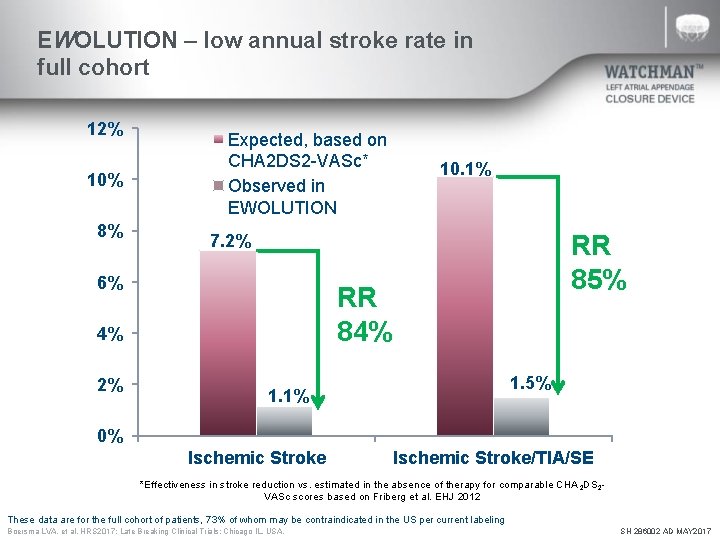
EWOLUTION – low annual stroke rate in full cohort 12% 10% 8% Expected, based on CHA 2 DS 2 -VASc* Observed in EWOLUTION RR 85% 7. 2% 6% RR 84% 4% 2% 10. 1% 1. 5% 1. 1% 0% Ischemic Stroke/TIA/SE *Effectiveness in stroke reduction vs. estimated in the absence of therapy for comparable CHA 2 DS 2 VASc scores based on Friberg et al. EHJ 2012 These data are for the full cohort of patients, 73% of whom may be contraindicated in the US per current labeling Boersma LVA, et al. HRS 2017; Late Breaking Clinical Trials: Chicago IL, USA. SH 286002 AD MAY 2017

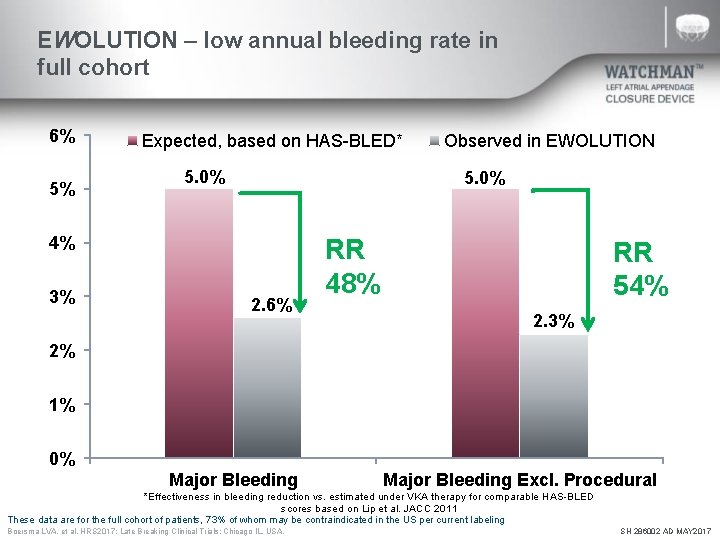
EWOLUTION – low annual bleeding rate in full cohort 6% 5% Expected, based on HAS-BLED* 5. 0% 4% 3% Observed in EWOLUTION 2. 6% RR 48% RR 54% 2. 3% 2% 1% 0% Major Bleeding Excl. Procedural *Effectiveness in bleeding reduction vs. estimated under VKA therapy for comparable HAS-BLED scores based on Lip et al. JACC 2011 These data are for the full cohort of patients, 73% of whom may be contraindicated in the US per current labeling Boersma LVA, et al. HRS 2017; Late Breaking Clinical Trials: Chicago IL, USA. SH 286002 AD MAY 2017

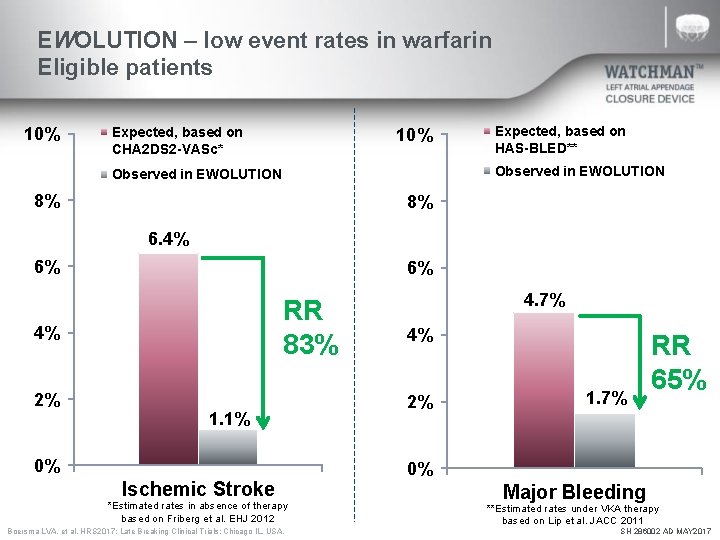
EWOLUTION – low event rates in warfarin Eligible patients 10% Expected, based on CHA 2 DS 2 -VASc* 10% Expected, based on HAS-BLED** Observed in EWOLUTION 8% 8% 6. 4% 6% 6% RR 83% 4% 2% 1. 1% 0% Ischemic Stroke *Estimated rates in absence of therapy based on Friberg et al. EHJ 2012 Boersma LVA, et al. HRS 2017; Late Breaking Clinical Trials: Chicago IL, USA. 4. 7% 4% 2% 1. 7% RR 65% 0% Major Bleeding **Estimated rates under VKA therapy based on Lip et al. JACC 2011 SH 286002 AD MAY 2017

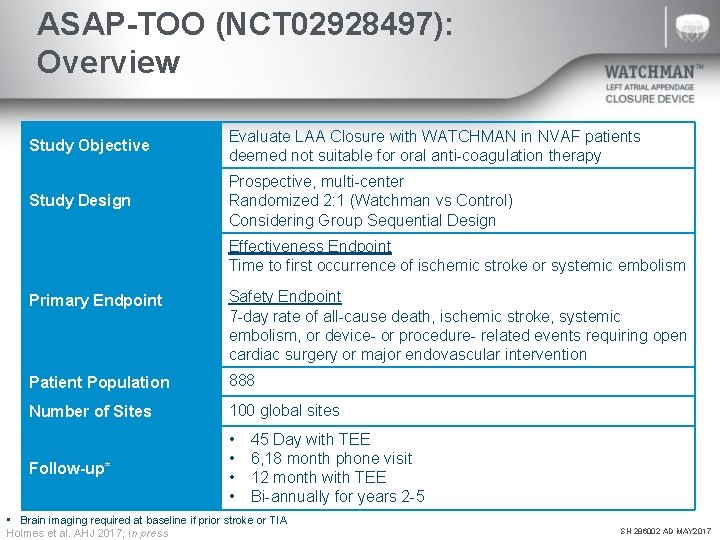
ASAP-TOO (NCT 02928497): Overview Study Objective Evaluate LAA Closure with WATCHMAN in NVAF patients deemed not suitable for oral anti-coagulation therapy Study Design Prospective, multi-center Randomized 2: 1 (Watchman vs Control) Considering Group Sequential Design Effectiveness Endpoint Time to first occurrence of ischemic stroke or systemic embolism Primary Endpoint Safety Endpoint 7 -day rate of all-cause death, ischemic stroke, systemic embolism, or device- or procedure- related events requiring open cardiac surgery or major endovascular intervention Patient Population 888 Number of Sites 100 global sites Follow-up* • • 45 Day with TEE 6, 18 month phone visit 12 month with TEE Bi-annually for years 2 -5 • Brain imaging required at baseline if prior stroke or TIA Holmes et al. AHJ 2017; in press SH 286002 AD MAY 2017

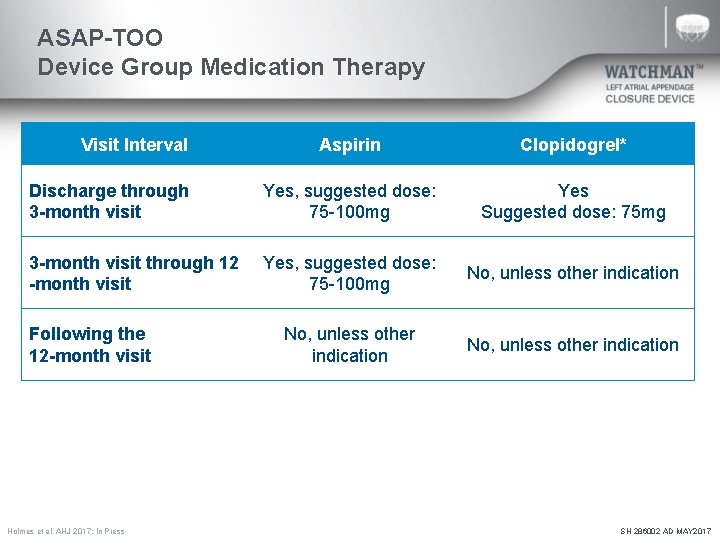
ASAP-TOO Device Group Medication Therapy Visit Interval Aspirin Clopidogrel* Discharge through 3 -month visit Yes, suggested dose: 75 -100 mg Yes Suggested dose: 75 mg 3 -month visit through 12 -month visit Yes, suggested dose: 75 -100 mg No, unless other indication Following the 12 -month visit *Clopidogrel may be substituted with ticagrelor or prasugrel if the subject requires the medication for other indications (e. g. acute coronary syndromes treated with drug eluting stents) or if the subject has a known resistance to clopidogrel. **Patients are allowed to be on dual antiplatelet therapy (outside of the protocol required 3 months period) if indicated due to a condition other than WATCHMAN implantation. Holmes et al. AHJ 2017; In Press SH 286002 AD MAY 2017

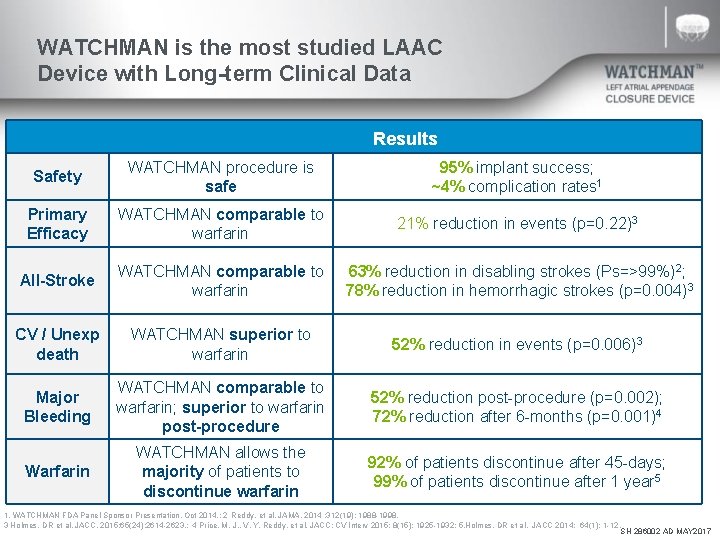
WATCHMAN is the most studied LAAC Device with Long-term Clinical Data Results Safety WATCHMAN procedure is safe 95% implant success; ~4% complication rates 1 Primary Efficacy WATCHMAN comparable to warfarin 21% reduction in events (p=0. 22)3 All-Stroke WATCHMAN comparable to 63% reduction in disabling strokes (Ps=>99%)2; warfarin 78% reduction in hemorrhagic strokes (p=0. 004)3 CV / Unexp death WATCHMAN superior to warfarin 52% reduction in events (p=0. 006)3 Major Bleeding WATCHMAN comparable to warfarin; superior to warfarin post-procedure 52% reduction post-procedure (p=0. 002); 72% reduction after 6 -months (p=0. 001)4 Warfarin WATCHMAN allows the majority of patients to discontinue warfarin 92% of patients discontinue after 45 -days; 99% of patients discontinue after 1 year 5 1. WATCHMAN FDA Panel Sponsor Presentation. Oct 2014. ; 2 Reddy, et al. JAMA. 2014 ; 312(19): 1988 -1998. 3 Holmes, DR et al. JACC. 2015; 65(24): 2614 -2623. ; 4 Price, M. J. , V. Y. Reddy, et al. JACC: CV Interv 2015; 8(15): 1925 -1932; 5. Holmes, DR et al. JACC 2014; 64(1): 1 -12. SH 286002 AD MAY 2017

WATCHMAN™ Clinical Leadership • WATCHMAN is a safe alternative to long-term warfarin therapy which offers comparable stroke risk reduction and enables patients to stop taking warfarin 1, 2 • WATCHMAN therapy demonstrated comparable stroke risk reduction and statistically superior reductions in major non-procedure related bleeding and cardiovascular death compared to warfarin 2, 4: • WATCHMAN demonstrated 95% implant success rate in both clinical and commercial setting 3, 5 – >92% warfarin cessation after 45 days, >99% after 1 year 1 1 Holmes, DR et al. JACC 2014; 64(1). 2 Holmes, DR et al. JACC 2015; 65(2). 3 Reddy VY, Holmes DR, et al. JACC 2016; Reddy, V. Y. , D. N. Gibson, et al. JACC 2017; 69(3). . 4 Price, M. J. , V. Y. Reddy, et al. JACC: CV Interv 2015; 8(15 ). 5 Reddy, V. Y. , D. N. Gibson, et al. JACC 2017; 69(3). SH 286002 AD MAY 2017

ABBREVIATED STATEMENT WATCHMANTM Left Atrial Appendage Closure Device with Delivery System and WATCHMAN Access System INDICATIONS FOR USE The WATCHMAN Device is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with non-valvular atrial fibrillation who: • Are at increased risk for stroke and systemic embolism based on CHADS 2 or CHA 2 DS 2 -VASc scores and are recommended for anticoagulation therapy; • Are deemed by their physicians to be suitable for warfarin; and • Have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin. The WATCHMAN Access System is intended to provide vascular and transseptal access for all WATCHMAN Left Atrial Appendage Closure Devices with Delivery Systems. CONTRAINDICATIONS Do not use the WATCHMAN Device if: • Intracardiac thrombus is visualized by echocardiographic imaging. • An atrial septal defect repair or closure device or a patent foramen ovale repair or closure device is present. • The LAA anatomy will not accommodate a device. See Table 46 in the DFU. • Any of the customary contraindications for other percutaneous catheterization procedures (e. g. , patient size too small to accommodate TEE probe or required catheters) or conditions (e. g. , active infection, bleeding disorder) are present. • There are contraindications to the use of warfarin, aspirin, or clopidogrel. • The patient has a known hypersensitivity to any portion of the device material or the individual components (see Device Description section) such that the use of the WATCHMAN Device is contraindicated. WARNINGS • Device selection should be based on accurate LAA measurements obtained using fluoro and ultrasound guidance (TEE recommended) in multiple angles (e. g. , 0º, 45º, 90º, 135º). • Do not release the WATCHMAN Device from the core wire if the device does not meet all release criteria. • If thrombus is observed on the device, warfarin therapy is recommended until resolution of thrombus is demonstrated by TEE. • The potential for device embolization exists with cardioversion <30 days following device implantation. Verify device position post-cardioversion during this period. • Administer appropriate endocarditis prophylaxis for 6 months following device implantation. The decision to continue endocarditis prophylaxis beyond 6 months is at physician discretion. • For single use only. Do not reuse, reprocess, or resterilize. PRECAUTIONS • The safety and effectiveness (and benefit-risk profile) of the WATCHMAN Device has not been established in patients for whom long-term anticoagulation is determined to be contraindicated. • The LAA is a thin-walled structure. Use caution when accessing the LAA and deploying the device. • Use caution when introducing the WATCHMAN Access System to prevent damage to cardiac structures. • Use caution when introducing the Delivery System to prevent damage to cardiac structures. • To prevent damage to the Delivery Catheter or device, do not allow the WATCHMAN Device to protrude beyond the distal tip of the Delivery Catheter when inserting the Delivery System into the Access Sheath. • If using a power injector, the maximum pressure should not exceed 100 psi. • In view of the concerns that were raised by the RE-ALIGN 1 study of dabigatran in the presence of prosthetic mechanical heart valves, caution should be used when prescribing oral anticoagulants other than warfarin in patients treated with the WATCHMAN Device. The WATCHMAN Device has only been evaluated with the use of warfarin post-device implantation. ADVERSE EVENTS Potential adverse events (in alphabetical order) which may be associated with the use of a left atrial appendage closure device or implantation procedure include but are not limited to: Air embolism, Airway trauma, Allergic reaction to contrast media/medications or device materials, Altered mental status, Anemia requiring transfusion, Anesthesia risks, Angina, Anoxic encephalopathy, Arrhythmias, Atrial septal defect , AV fistula , Bruising, hematoma or seroma, Cardiac perforation , Chest pain/discomfort, Confusion post procedure, Congestive heart failure, Contrast related nephropathy, Cranial bleed, Decreased hemoglobin, Deep vein thrombosis, Death, Device embolism, Device fracture, Device thrombosis, Edema, Excessive bleeding, Fever, Groin pain, Groin puncture bleed, Hematuria, Hemoptysis, Hypotension, Hypoxia, Improper wound healing, Inability to reposition, recapture, or retrieve the device, Infection / pneumonia, Interatrial septum thrombus, Intratracheal bleeding, Major bleeding requiring transfusion, Misplacement of the device / improper seal of the appendage / movement of device from appendage wall, Myocardia erosion, Nausea, Oral bleeding, Pericardial effusion / tamponade, Pleural effusion, Prolonged bleeding from a laceration, Pseudoaneurysm, Pulmonary edema, Renal failure, Respiratory insufficiency / failure, Surgical removal of the device, Stroke – Ischemic , Stroke – Hemorrhagic, Systemic embolism, TEE complications (throat pain, bleeding, esophageal trauma), Thrombocytopenia, Thrombosis, Transient ischemic attack (TIA), Valvular damage, Vasovagal reactions There may be other potential adverse events that are unforeseen at this time. CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the complete “Directions for Use” for more information on Indications, Contraindications, Warnings, Precautions, Adverse Events, and Operator’s Instructions. © 2015 Boston Scientific Corporation or its affiliates. All rights reserved. 1 Eikelboom JW, Connolly SJ, Brueckmann M, et al. N Engl J Med 2013; 369: 1206 -14. SH 286002 AD MAY 2017