Pascals Principle The pressure applied to any surface

















- Slides: 20

Pascal’s Principle – The pressure applied to any surface of a confined fluid is transmitted equally in every direction throughout the fluid.

Blaise Pascal. Christian Came up with first calculating machine. Euclidean Geometry

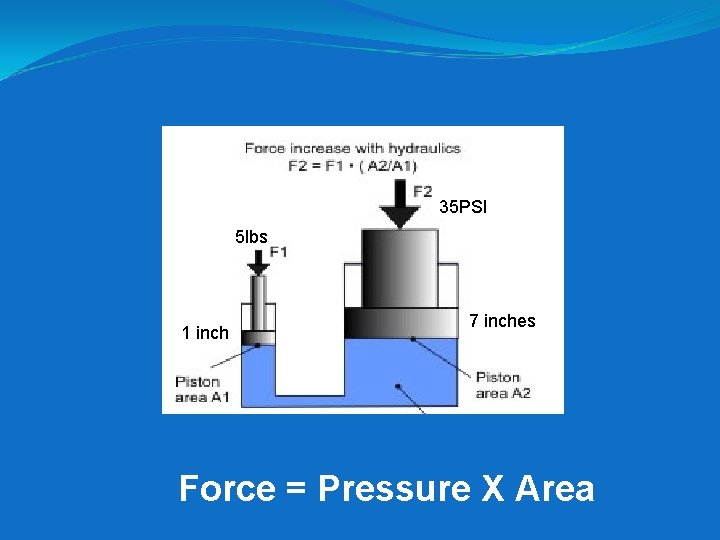
Hydraulic Press – One of the most important applications of Pascal’s Principle.

35 PSI 5 lbs 1 inch 7 inches Force = Pressure X Area

A small cylinder of a hydraulic press has an area of 3 in 2. If a force of 6 pounds is applied to the small piston, how much is the pressure within the larger cylinder increased? = 6 pounds / 3 in 2 2 psi

The large cylinder of a hydraulic press has an area of 50 in 2. What will be the force upon it if a pressure of 10 psi is produced by the small piston? = 10 psi x 50 in 2 500 pounds

Robert Boyle Christian that helped fund the translation of the Bible. Improved vacuum pump Father of Modern Day Chemistry.

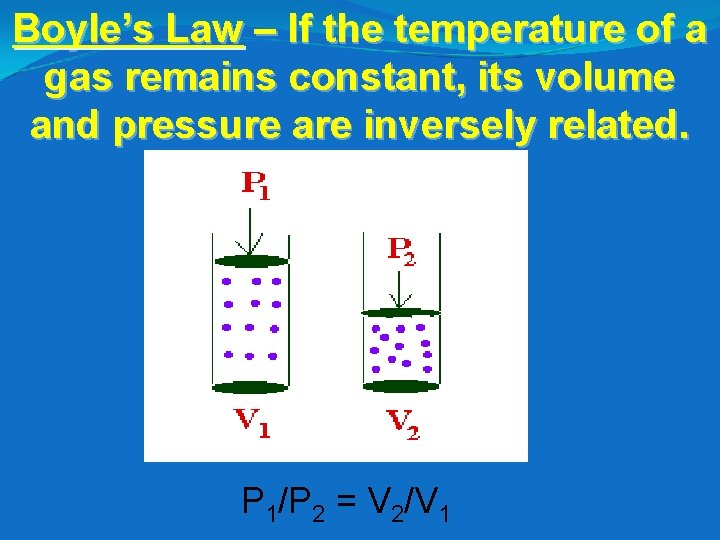
Boyle’s Law – If the temperature of a gas remains constant, its volume and pressure are inversely related. P 1/P 2 = V 2/V 1

If a pressure of 20 psi on a volume of 10 in 3 is reduced to 4 psi, by what factor does the pressure change? 20 psi/4 psi = 5

If the pressure of a gas triples, how does the volume of the gas change? Since pressure and volume are inversely related, it decreases by the inverse. The inverse of 3/1 is 1/3.

If the volume of a gas triples, how does the pressure of the gas change? Since pressure and volume are inversely related, it decreases by the inverse. The inverse of 3/1 is 1/3.

If a mass of air in a basketball has a pressure of 30 psi and a volume of 20 in 3 , what will be the new pressure if it is compressed to a new volume of 10 in 3 ? 30 psi / P 2 = 20 in 3 / 10 in 3 Solve for P 2 = 60 PSI

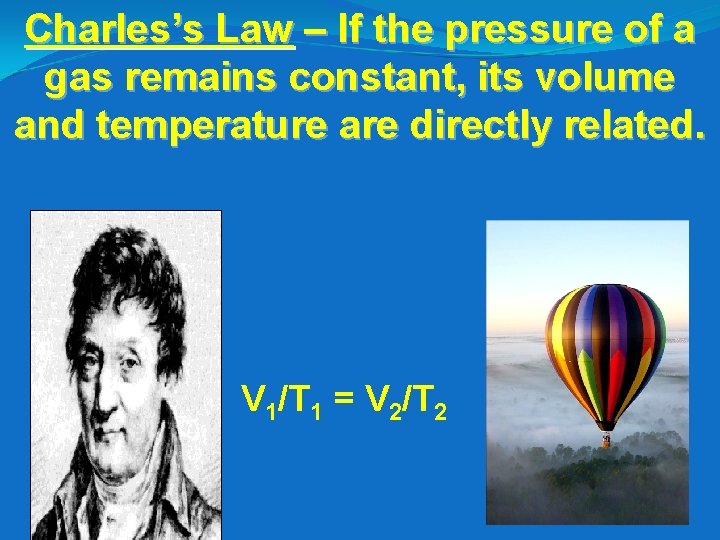
Charles’s Law – If the pressure of a gas remains constant, its volume and temperature are directly related. V 1/T 1 = V 2/T 2

Combined Gas Law– If the volume of a gas remains constant, its pressure and temperature are directly related. P 1/T 1 = P 2/T 2

If the volume of a gas is tripled, how does the absolute temperature of the gas change? Since temperature and volume are directly related, it is also tripled.

If the absolute temperature of 12 in 3 of a gas decreases by a factor of 2, what will be the new volume? Since temperature and volume are directly related it will also decrease by a factor of 2. 12 in 3 / 2 = 6 in 3

If the pressure of a gas is tripled, how does the absolute temperature of the gas change? Since temperature and pressure are directly related, it is also tripled.

If the absolute temperature of a gas with a pressure of 20 psi increases by a factor of 2, what will be the new pressure? Since temperature and pressure are directly related it will also increase by a factor of 2. 20 psi x 2 = 40 psi

Absolute Zero - The point at which molecules have no heat energy and therefore do not move. This is the lowest possible temperature. -273. 15 Celsius, -459. 67 Fahrenheit.

Kelvin Scale - The scale of absolute temperature. Absolute Zero is 0 Kelvin. K = Celsius + 273. 15 Use K reading in equation for Charles’ Law