OpioidInduced Hyperalgesia Mary Lynn Mc Pherson Pharm D





















- Slides: 31

Opioid-Induced Hyperalgesia Mary Lynn Mc. Pherson, Pharm. D. , MA, BCPS, CPE Professor University of Maryland School of Pharmacy Executive Director, Post-Graduate Education in Palliative Care Hospice and Palliative Care Consultant Pharmacist

Meet Mr. P. v 38 year old Caucasian male with an admitting hospice diagnosis of sepsis v Previous work accident resulting in “internal decapitation” with rod placed; left arm “internal amputation” with rods place; ear amputation with reattachment attempts. Patient quadriplegic. v History of MI, CVA, PEG placement and removal, seizures, extensor tone spasms (that bent cervical titanium rod in cervical spine), chronic neuropathic pain, sacral wound. 2

Meet Mr. P. v History of encephalopathy, aspiration pneumonia, osteomyelitis, GI and GU bleeding, neurogenic bladder, asthma, chronic anemia, hand contractures, bilateral foot drop, anxiety, severe depression, progressive chronic debility, multiple episodes septic shock with hospitalization. v Recently elected comfort care only (no further admissions). 3

Mrs. P. says… v She is upset because her husband has little time left, and they have three small children v He sits in a recliner, just looking out at the front yard v She is very worried about new plan of care because the patient has “excruciating pain when he becomes septic…he screams until he becomes hoarse from the pain. ” v She has experienced this for the past 5 years. v Patient a complete assist for all ADLs. v Patient 72”, weight 170 pounds, BMI 23, PPS 20% 4

Analgesics for Mr. P. on Hospice Admission v Implanted pump delivering baclofen v Transdermal fentanyl 150 mcg/hour v Transitioned from oral morphine 750 mg per day to morphine infusion, and titrated to 40 mg/hour continuous IV infusion (RN then called pharmacist) v Patient hypervigilant, twitching, screaming in pain (could hear him screaming over the phone), says it hurts EVERYWHERE v What’s going on? 5

What’s going on with Mr. P? A. His pain abruptly escalated B. He has developed opioid-induced hyperalgesia C. He is experiencing opioid intoxication D. He is attention-seeking 6

Opioid Therapy • Cornerstone of therapy for the treatment of moderate to severe pain (cancer, non-cancer) • Increased availability and comfort level in using opioids in recent years – Greater attention to pain management – Better education – Used earlier in disease process – Used in higher doses 7

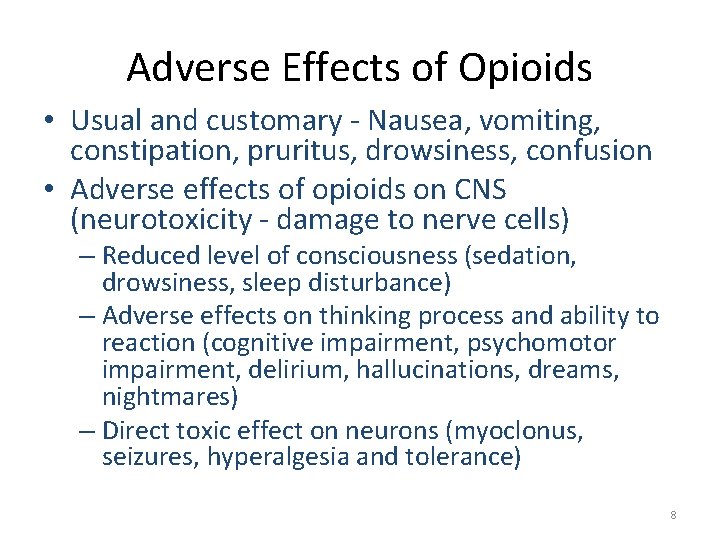
Adverse Effects of Opioids • Usual and customary - Nausea, vomiting, constipation, pruritus, drowsiness, confusion • Adverse effects of opioids on CNS (neurotoxicity - damage to nerve cells) – Reduced level of consciousness (sedation, drowsiness, sleep disturbance) – Adverse effects on thinking process and ability to reaction (cognitive impairment, psychomotor impairment, delirium, hallucinations, dreams, nightmares) – Direct toxic effect on neurons (myoclonus, seizures, hyperalgesia and tolerance) 8

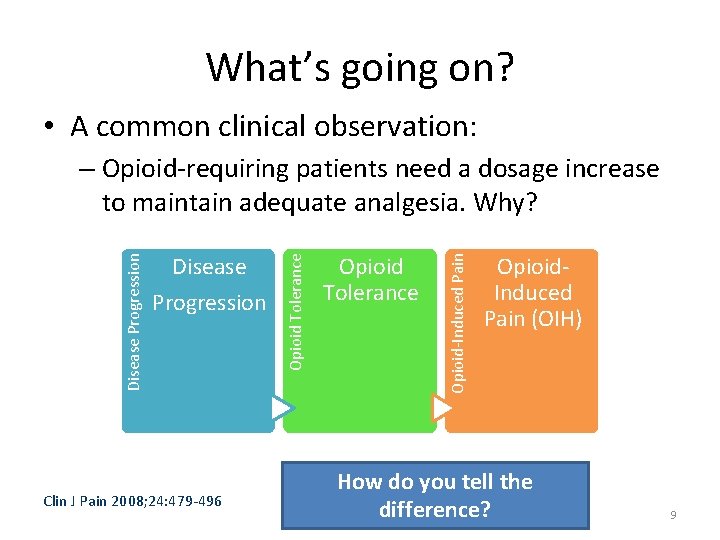
What’s going on? • A common clinical observation: Clin J Pain 2008; 24: 479 -496 Opioid Tolerance Opioid-Induced Pain Disease Progression Opioid Tolerance Disease Progression – Opioid-requiring patients need a dosage increase to maintain adequate analgesia. Why? Opioid. Induced Pain (OIH) How do you tell the difference? 9

Opioid-Induced Hyperalgesia • A state of nociceptive sensitization caused by exposure to opioids • Results in a paradoxical adverse response to opioid therapy • Patient receiving opioids becomes more sensitive to painful stimulation – This increased sensitivity to pain is a new, unique entity that is distinct from patient’s original underlying painful condition • Allodynia, more diffuse, expanded area 10

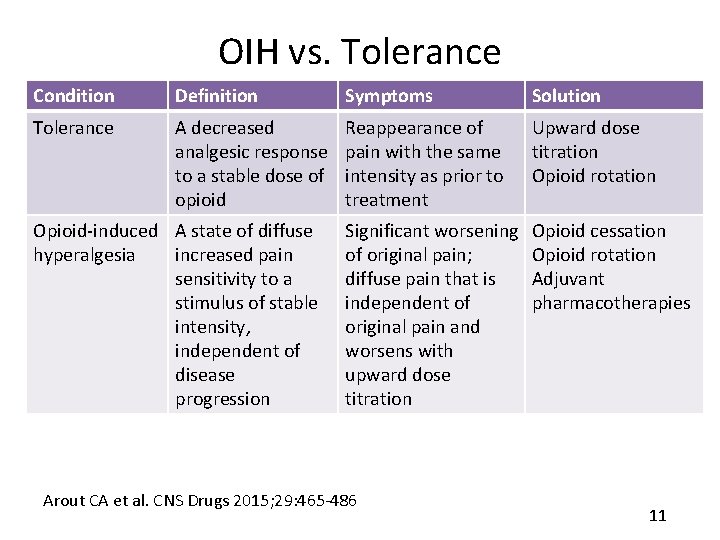
OIH vs. Tolerance Condition Definition Symptoms Solution Tolerance A decreased analgesic response to a stable dose of opioid Reappearance of pain with the same intensity as prior to treatment Upward dose titration Opioid rotation Significant worsening of original pain; diffuse pain that is independent of original pain and worsens with upward dose titration Opioid cessation Opioid rotation Adjuvant pharmacotherapies Opioid-induced A state of diffuse hyperalgesia increased pain sensitivity to a stimulus of stable intensity, independent of disease progression Arout CA et al. CNS Drugs 2015; 29: 465 -486 11

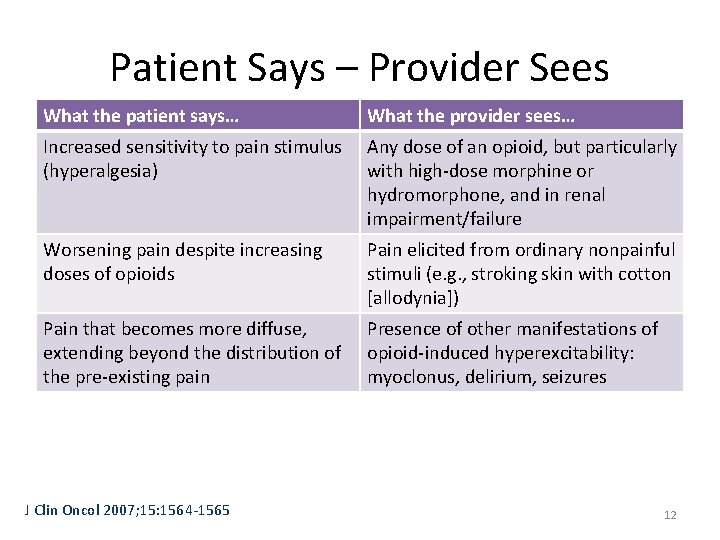
Patient Says – Provider Sees What the patient says… What the provider sees… Increased sensitivity to pain stimulus (hyperalgesia) Any dose of an opioid, but particularly with high-dose morphine or hydromorphone, and in renal impairment/failure Worsening pain despite increasing doses of opioids Pain elicited from ordinary nonpainful stimuli (e. g. , stroking skin with cotton [allodynia]) Pain that becomes more diffuse, extending beyond the distribution of the pre-existing pain Presence of other manifestations of opioid-induced hyperexcitability: myoclonus, delirium, seizures J Clin Oncol 2007; 15: 1564 -1565 12

How common is this? • Overall prevalence indeterminant – Clinical trials vs. thermal/cold stimulation – Populations studied (healthy volunteers, acute pain, chronic pain, cancer pain, opioid-use disorder – Does not occur in all patients • 14% of 81 cancer patientsa – Diffuse pain; not explained by history or disease progression – Unresponsive to morphine titration; alleviated by rotation to methadone • 28% of 197 chronic pain patientsb a. Mercadante et al. Ann Palliat Med 2012; 1(1): 10 -3 b. Ackerman J Ky Med Assoc 2006; 104(9): 419 -412 13

Neurobiology of OIH v OIH occurs independently of opioid receptors • Not a consequence of either prior or concurrent opioid receptor activity v Central sensitization in OIH v Neuroimmune mechanisms v Neurotransmitter mechanisms Arout CA et al. CNS Drugs 2015; 29: 465 -486 14

Neurobiology of OIH v Central sensitization in OIH • Allodynia, hyperalgesia, expansion of receptive field → secondary hyperalgesia • Sensitization refers to ↑ synaptic transmission and pain hypersensitivity, reduced nociceptive thresholds, phenotypic expression of pain Arout CA et al. CNS Drugs 2015; 29: 465 -486 15

Neurobiology of OIH v Central sensitization in OIH • OIH may result from sensitization of primary afferent neurons and microglia → neuroplastic changes of receptor systems and increased excitatory neurotransmission → increased neurotransmitters such as glutamate and substance P, sensitization of pain pathways → amplified subjective nociceptive responses • Descending facilitation of nociception from the rostroventral medulla implicated in OIH Arout CA et al. CNS Drugs 2015; 29: 465 -486 16

Neurobiology of OIH v Neuroimmune Mechanisms • Microglia activation → reduces analgesic efficacy of opioids (activate TLR 4 receptor) • Release of pro-inflammatory cytokines (TNF-α, IL 1β, IL-6), chemokines, lipid mediators of inflammation, matrix metalloproteases, excitatory amino acids, and nitric oxide • Increased number and conductance of AMPA and NMDA receptors • Down regulation of GABA receptors Arout CA et al. CNS Drugs 2015; 29: 465 -486 17

Neurobiology of OIH v Neurotransmitter Mechanisms • Glutamate • Endogenous ligand for NMDA receptor • GABA • Down regulated with chronic opioid use v Opioid Metabolites • Morphine-3 -glucuronide • Less clear Arout CA et al. CNS Drugs 2015; 29: 465 -486 18

Morphine and Hydromorphone Active Metabolite Accumulation in Renal Failure http: //palliative. info/pages/Teaching. Material. htm; Used with permission 19

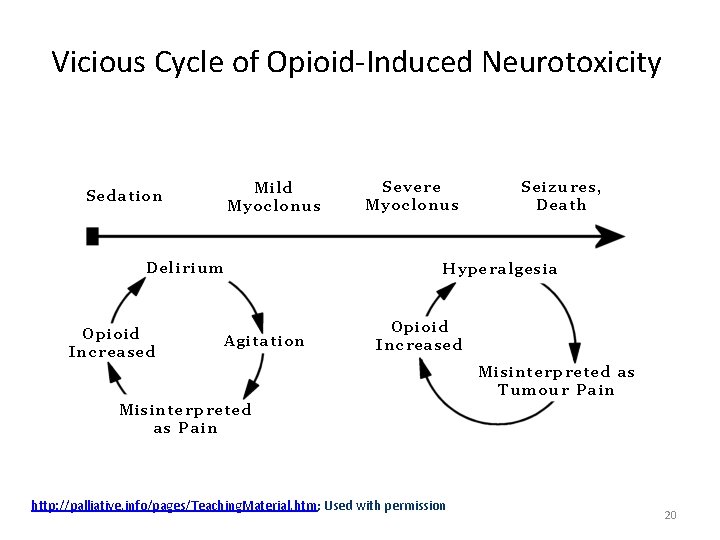
Vicious Cycle of Opioid-Induced Neurotoxicity http: //palliative. info/pages/Teaching. Material. htm; Used with permission 20

What do we do now? 21

Management of OIH • Hydration if clinically appropriate • Reduce the opioid dose – Consider use of an opioid-sparing coanalgesic • Opioid rotation – Allows comparable analgesia at a lower equianalgesic dose – Fentanyl, methadone • NMDA receptor antagonist – Ketamine, dextromethorphan • • Gabapentinoids Propranolol Cyclooxygenase inhibitors Future – glial targets, cannabinoids, neurosteroids Clin J Pain 2008; 24: 479 -496; Arout CA et al. CNS Drugs 2015; 29: 465 -486 22

Ketamine and Methadone v Ketamine - Potent non-competitive NMDA receptor antagonist • Pain relief due to analgesia or antihyperalgesia? • Activity due to metabolite – norketamine? v Peri-operative administration of low-dose ketamine may reduce OIH or analgesic tolerance v Research shown prevented or reduced postoperative hyperalgesia and morphine consumption in patients who received remifentanil v Methadone Arout CA et al. CNS Drugs 2015; 29: 465 -486; Axelrod DJ et al. J Opioid Manage 2007; 3: 113 -114 23

Gabapentinoids • “Gabapentin improves cold-pressor pain responses in methadone-maintained patients” – MM patients – demonstrated to be hyperalgesic to cold-pressor pain • Gabapentin 2400 mg/day, 5 week trial – Significance seen in change in cold-pressor pain threshold and tolerance • At both peak and trough methadone levels – Gabapentin effective in decreasing OIH in patients who are abstinent and stable in MM treatment Compton P et al. Drug Alcohol Depend 2010; 109(1 -3): 213 -9. 24

Gabapentinoids • Pregabalin – Single dose given pre-operatively (intra-operative remifentanil) → attenuated OIHa – Pregabalin, ketamine, pregabalin + ketamine, placebo administered prior to hip surgeryb • Both ketamine alone and pregabalin alone reduced post-operative morphine requirements • Combination further reduced morphine requirements a. Lee C et al. Korean J Anesthesiol 2013; 64(1): 19 -24 b. Martinez V et al. Anaesthesia 2014; 69(1): 46 -52 25

Suggested Clinical Criteria for Diagnosing OIH • Increased pain intensity during ongoing opioid treatment • No evidence for underlying disease progression. • No evidence for either clinical or pharmacological opioid withdrawal (i. e. , symptoms and signs of opioid withdrawal; increased pain as a result of end of previous opioid dose effect). • No evidence for opioid tolerance: to be tested clinically be decreased pain in response to an adequate opioid rescue dose. • Decrease in pain intensity in response to a reduction in opioid dose (gradual dose reduction might be required to avoid abstinence syndrome). • No evidence of addictive behavior. Eisenberg E et al. J Pain Symptom Manage 2015; 49(3): 632 -636 26

Back at the ranch with Mr. P. v Implanted pump delivering baclofen (“state-allowed maximum dose”) v Transdermal fentanyl 150 mcg/hour v Transitioned from oral morphine 750 mg per day to morphine infusion, and titrated to 40 mg/hour continuous IV infusion (RN then called pharmacist) v What’s the plan? 27

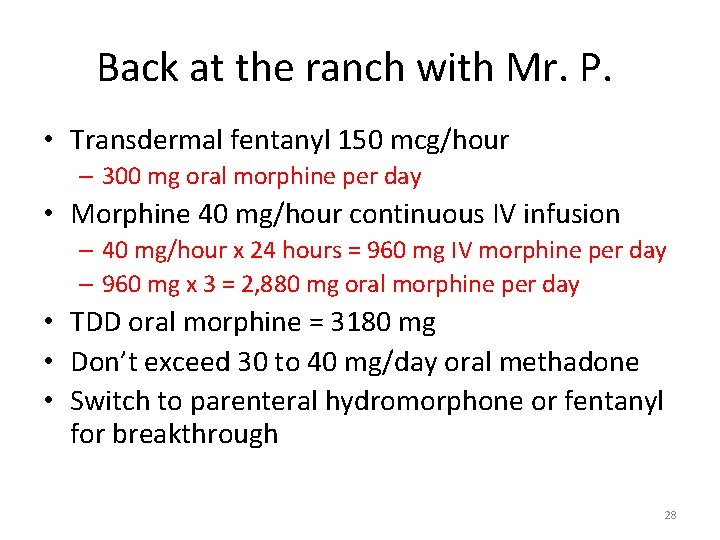
Back at the ranch with Mr. P. • Transdermal fentanyl 150 mcg/hour – 300 mg oral morphine per day • Morphine 40 mg/hour continuous IV infusion – 40 mg/hour x 24 hours = 960 mg IV morphine per day – 960 mg x 3 = 2, 880 mg oral morphine per day • TDD oral morphine = 3180 mg • Don’t exceed 30 to 40 mg/day oral methadone • Switch to parenteral hydromorphone or fentanyl for breakthrough 28

Key Takeaways v. Key Takeaway #1 • Be ALERT for clinical indicators suggestive of OIH v. Key Takeaway #2 • Consider simple management strategies – hydration, opioid-sparing strategies, opioid rotation to another opioid v. Key Takeaway #3 • Consider secondary management strategies – rotation to methadone, NMDA receptor antagonist 29

v “At such time, I have certainly felt it a great responsibility to say that pain, which I know is an evil, is less injurious than morphia, which may be an evil. v I have much reason to suspect that a reliance upon hypodermic morphia only ended in a curious state of perpetuated pain. ” » Dr. T. Clifford Albutt » 1870 English physician chronicling first documented observation of increased pain sensitivity from opioid exposure. Practitioner 1870; 3: 327 -330. 30

Opioid-Induced Hyperalgesia Mary Lynn Mc. Pherson, Pharm. D. , MA, BCPS, CPE Professor University of Maryland School of Pharmacy Executive Director, Post-Graduate Education in Palliative Care Hospice and Palliative Care Consultant Pharmacist
 Pain pathophysiology
Pain pathophysiology Visceral hyperalgesia mayo clinic
Visceral hyperalgesia mayo clinic Pherson associates
Pherson associates Scott mc pherson
Scott mc pherson Mary lynn manns
Mary lynn manns Mary lynn manns
Mary lynn manns Hpc exf
Hpc exf Outfield pharm
Outfield pharm Pharmlinks
Pharmlinks Noel pharm
Noel pharm Pharm
Pharm Letazol
Letazol Bachelor of pharmaceutical science advanced with honours
Bachelor of pharmaceutical science advanced with honours Pharm gkb
Pharm gkb Library.med.utah.edu/kw/pharm/hyper heart.html
Library.med.utah.edu/kw/pharm/hyper heart.html Pharm id
Pharm id Pharm406
Pharm406 Pharm
Pharm Secur pharm
Secur pharm Library.med.utah.edu/kw/pharm/hyper heart.html
Library.med.utah.edu/kw/pharm/hyper heart.html Peptide api manufacturing
Peptide api manufacturing Bc bio-pharm
Bc bio-pharm Mary wollstonecraft mary a fiction
Mary wollstonecraft mary a fiction Steven benson murder
Steven benson murder Quentin staples
Quentin staples Lynn university library hours
Lynn university library hours Lynn mandeltort
Lynn mandeltort Lynn brewer enron
Lynn brewer enron Lynn grainger
Lynn grainger Judge donnelly cook county
Judge donnelly cook county How many siblings did loretta lynn have
How many siblings did loretta lynn have Lynn lawrence optometry
Lynn lawrence optometry





















































