Octaplas LG An alternative to fresh frozen plasma









- Slides: 31

Octaplas. LG - An alternative to fresh frozen plasma FSAIO´s Efterårsfagdag, October 6, 2017 Andrea Neisser-Svae, MSc, Ph. D Product Expert ICEM Octapharma

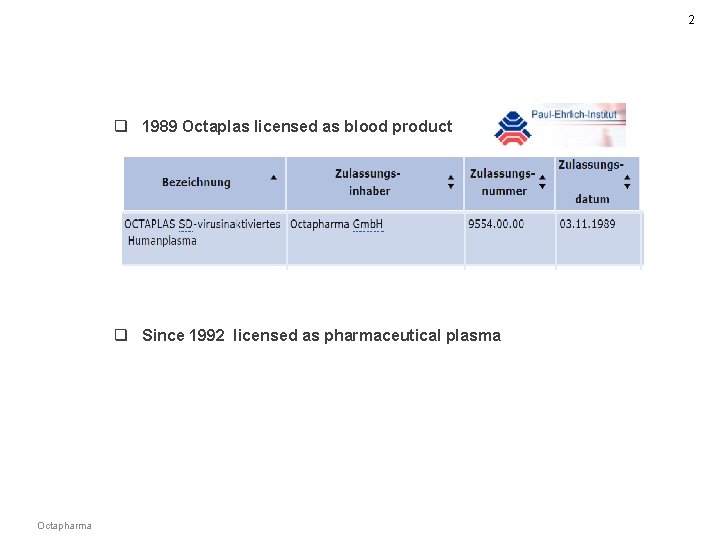
2 q 1989 Octaplas licensed as blood product q Since 1992 licensed as pharmaceutical plasma Octapharma

3 Octaplas(LG) worldwide In 48 Octapharma countries (1992 – 2016)

4 Requirements a therapeutic plasma should fulfill of pathogen q Reduced risk for pathogentransmission of side q Reduced risk for sideeffects (e. g. TRALI and allergic reactions) q Reduced bad-to-bag variance Coagulationfactorand andinhibitor content known q Coagulation Octapharma

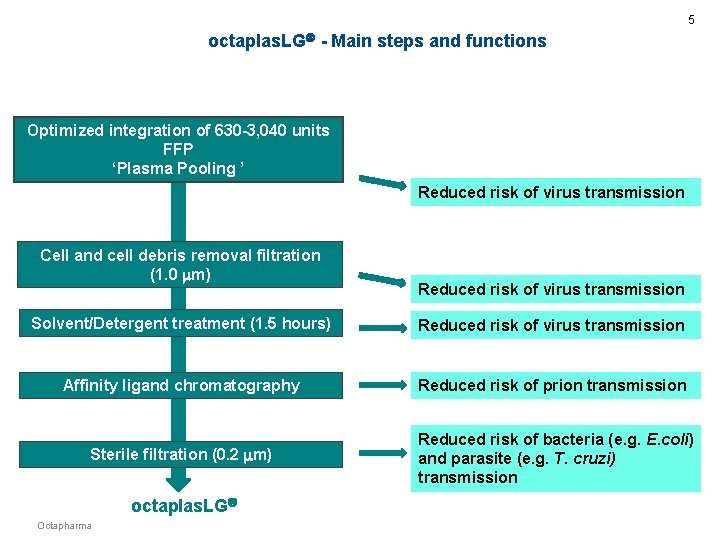
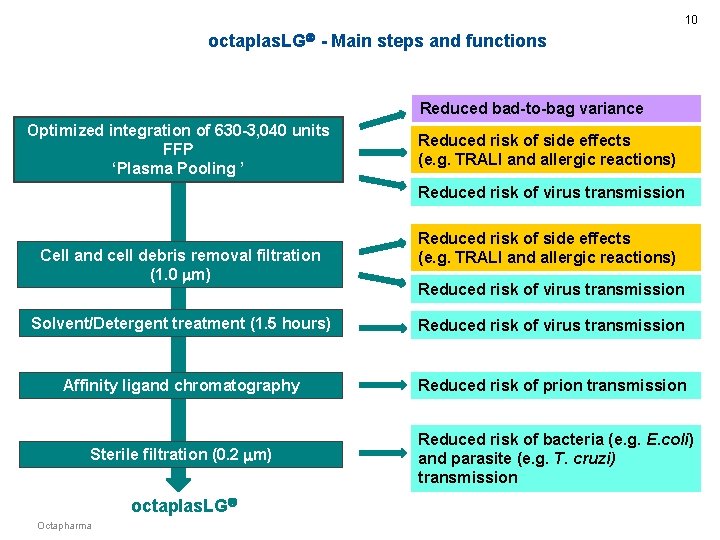
5 octaplas. LG - Main steps and functions Optimized integration of 630 -3, 040 units FFP ‘Plasma Pooling ’ Reduced risk of virus transmission Cell and cell debris removal filtration (1. 0 m) Reduced risk of virus transmission Solvent/Detergent treatment (1. 5 hours) Reduced risk of virus transmission Affinity ligand chromatography Reduced risk of prion transmission Sterile filtration (0. 2 m) Reduced risk of bacteria (e. g. E. coli) and parasite (e. g. T. cruzi) transmission octaplas. LG Octapharma

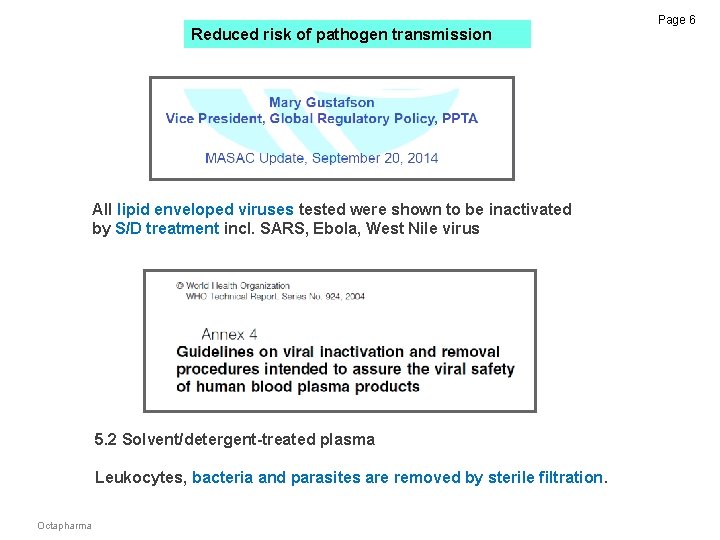
Reduced risk of pathogen transmission All lipid enveloped viruses tested were shown to be inactivated by S/D treatment incl. SARS, Ebola, West Nile virus 5. 2 Solvent/detergent-treated plasma Leukocytes, bacteria and parasites are removed by sterile filtration. Octapharma Page 6

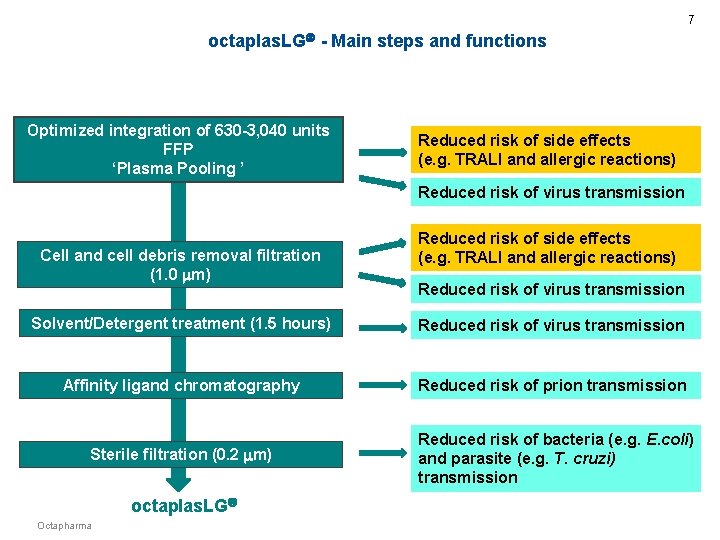
7 octaplas. LG - Main steps and functions Optimized integration of 630 -3, 040 units FFP ‘Plasma Pooling ’ Reduced risk of side effects (e. g. TRALI and allergic reactions) Reduced risk of virus transmission Cell and cell debris removal filtration (1. 0 m) Reduced risk of side effects (e. g. TRALI and allergic reactions) Reduced risk of virus transmission Solvent/Detergent treatment (1. 5 hours) Reduced risk of virus transmission Affinity ligand chromatography Reduced risk of prion transmission Sterile filtration (0. 2 m) Reduced risk of bacteria (e. g. E. coli) and parasite (e. g. T. cruzi) transmission octaplas. LG Octapharma

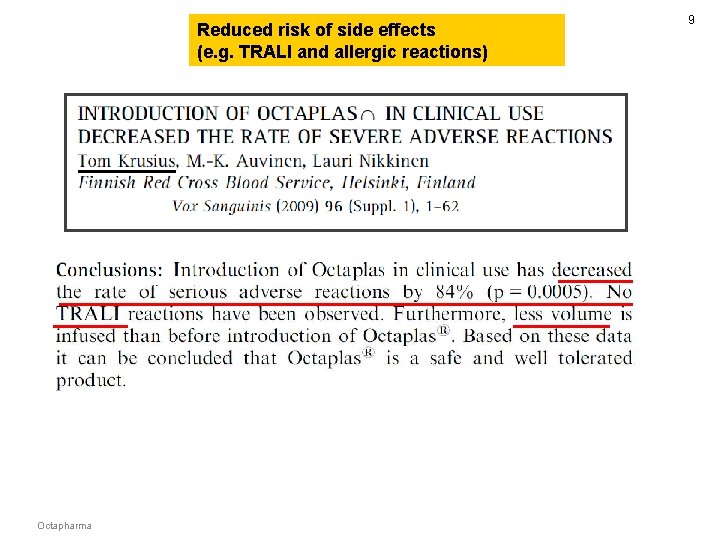
8 Reduced risk of side effects (e. g. TRALI and allergic reactions) FFP 1, 135 bags Octaplas 2, 621 bags Octapharma

Reduced risk of side effects (e. g. TRALI and allergic reactions) Octapharma 9

10 octaplas. LG - Main steps and functions Reduced bad-to-bag variance Optimized integration of 630 -3, 040 units FFP ‘Plasma Pooling ’ Reduced risk of side effects (e. g. TRALI and allergic reactions) Reduced risk of virus transmission Cell and cell debris removal filtration (1. 0 m) Reduced risk of side effects (e. g. TRALI and allergic reactions) Reduced risk of virus transmission Solvent/Detergent treatment (1. 5 hours) Reduced risk of virus transmission Affinity ligand chromatography Reduced risk of prion transmission Sterile filtration (0. 2 m) Reduced risk of bacteria (e. g. E. coli) and parasite (e. g. T. cruzi) transmission octaplas. LG Octapharma

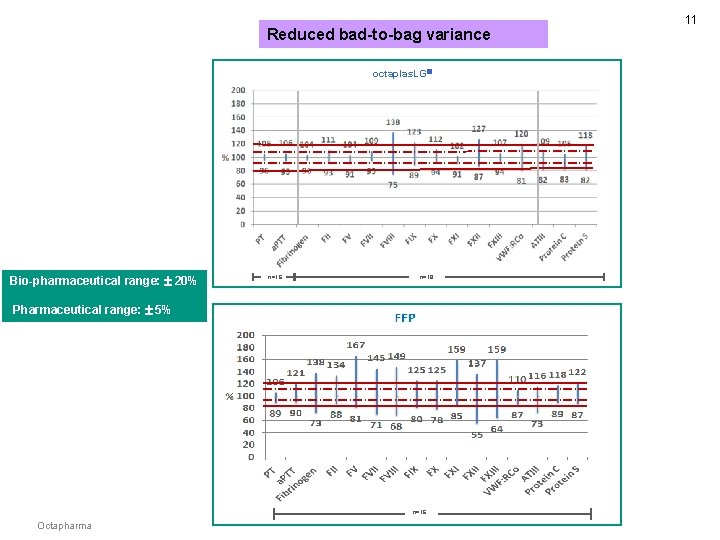
Reduced bad-to-bag variance octaplas. LG Bio-pharmaceutical range: 20% n=16 n=18 Pharmaceutical range: 5% n=16 Octapharma 11

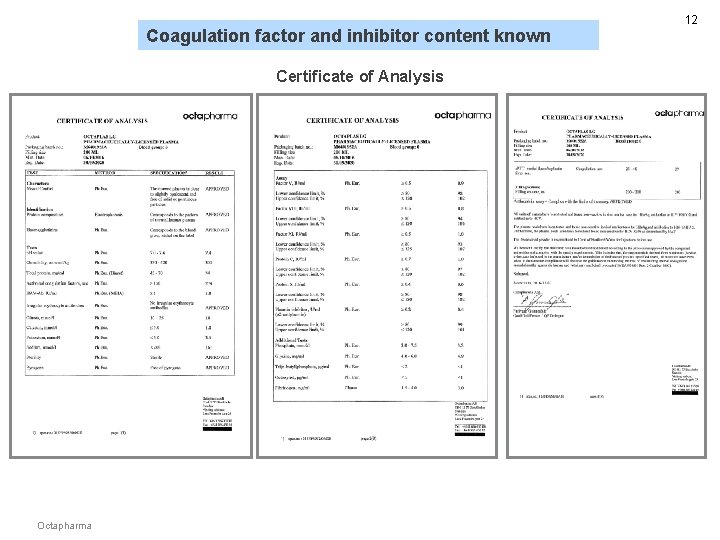
Coagulation factor and inhibitor content known Certificate of Analysis Octapharma 12

13 Every batch is tested and released internal as well as by an authority Octapharma

14 27 years of clinical experience More than 13. 5 million bags have been transfused to more than 4. 5 million patients in different indications Octapharma

15 Published clinical experiences with Octaplas(LG) Adult Patients Indication TTP Cardiac surgery Liver disease Transplantation (heard, liver, lung) Major surgery Obstetric and gynaecological emergencies Trauma Coagulation disorders Octapharma

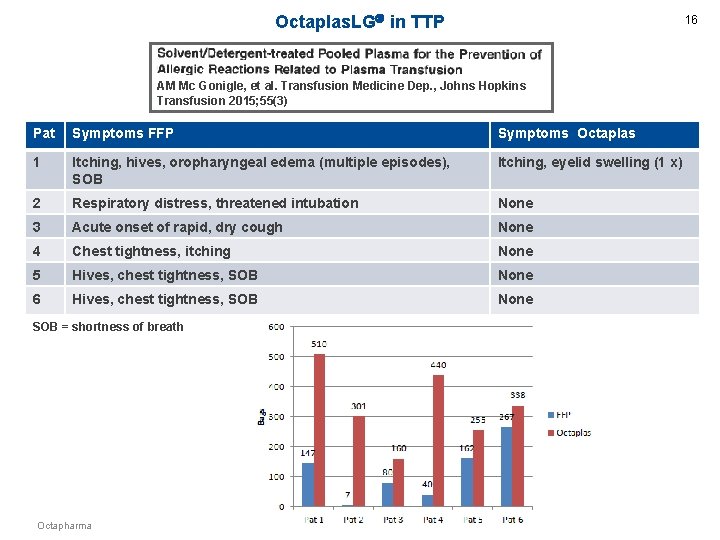
Octaplas. LG in TTP 16 AM Mc Gonigle, et al. Transfusion Medicine Dep. , Johns Hopkins Transfusion 2015; 55(3) Pat Symptoms FFP Symptoms Octaplas 1 Itching, hives, oropharyngeal edema (multiple episodes), SOB Itching, eyelid swelling (1 x) 2 Respiratory distress, threatened intubation None 3 Acute onset of rapid, dry cough None 4 Chest tightness, itching None 5 Hives, chest tightness, SOB None 6 Hives, chest tightness, SOB None SOB = shortness of breath Octapharma

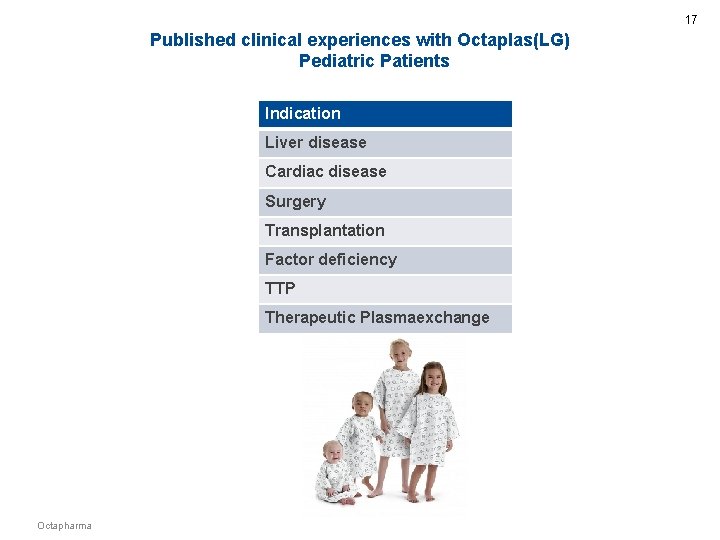
17 Published clinical experiences with Octaplas(LG) Pediatric Patients Indication Liver disease Cardiac disease Surgery Transplantation Factor deficiency TTP Therapeutic Plasmaexchange Octapharma

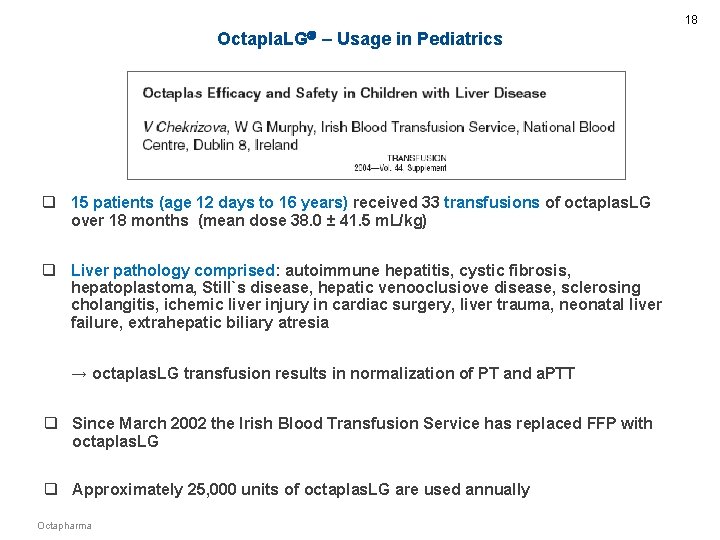
18 Octapla. LG – Usage in Pediatrics q 15 patients (age 12 days to 16 years) received 33 transfusions of octaplas. LG over 18 months (mean dose 38. 0 ± 41. 5 m. L/kg) q Liver pathology comprised: autoimmune hepatitis, cystic fibrosis, hepatoplastoma, Still`s disease, hepatic venooclusiove disease, sclerosing cholangitis, ichemic liver injury in cardiac surgery, liver trauma, neonatal liver failure, extrahepatic biliary atresia → octaplas. LG transfusion results in normalization of PT and a. PTT q Since March 2002 the Irish Blood Transfusion Service has replaced FFP with octaplas. LG q Approximately 25, 000 units of octaplas. LG are used annually Octapharma

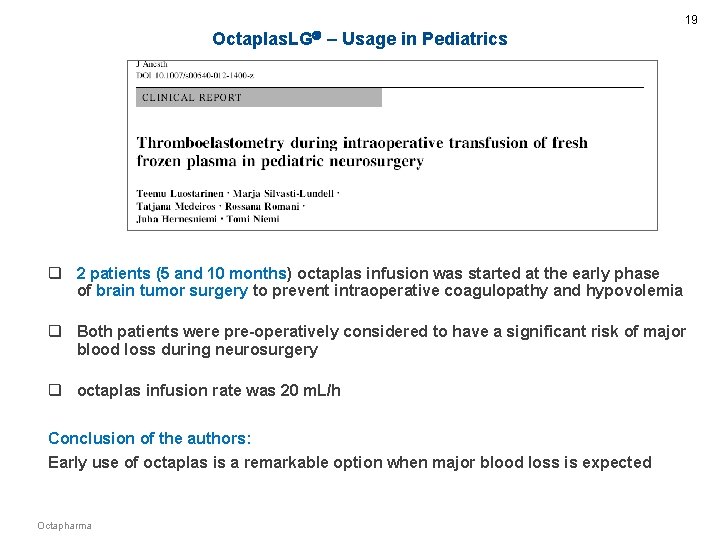
19 Octaplas. LG – Usage in Pediatrics q 2 patients (5 and 10 months) octaplas infusion was started at the early phase of brain tumor surgery to prevent intraoperative coagulopathy and hypovolemia q Both patients were pre-operatively considered to have a significant risk of major blood loss during neurosurgery q octaplas infusion rate was 20 m. L/h Conclusion of the authors: Early use of octaplas is a remarkable option when major blood loss is expected Octapharma

20 Octaplas. LG – Usage in Pediatrics q In 30 patients (9 months to 22 years) octaplas. LG was used in 282 TPE q Indications: TTP, Myasthenia gravis, Opsoklonus Myoklonus syndrome, Sepsis/SIRS, ITP, MTXintoxication, Hyper-Ig. E syndrome, Dysproteinemia, autoimmune hemolytic anemia, autoimmune disease, rhabdomyolysis Conclusion of the authors: The use of octaplas. LG as plasma exchange fluid was safe and efficient to stabilize the coagulation parameters. We would recommend to use in children who are at risk for bleeding instead of Albumin alone at least Albumin plus octaplas. Octapharma

21 Octaplas. LG – Usage in Pediatrics BJH 2013; 161: 1 -81 q In 29 patients (1 day to 15 years) octaplas was used in 51 infusions (total 111 units) q Main diagnoses: - Cardiac defects (45%) incl. cardiopulmonary bypass surgery, - Liver dysfunction (20%) - Infusions (27. 5%) for transplant-associated thrombotic microangiopathy, which is considered one of the most severe complications after hematopoietic stem cell transplantation. Conclusion of the authors No adverse reactions were recorded from any of the 51 infusion episodes. In our experience to date (2013), Octaplas is safe and well tolerated in the pediatric population. Octapharma

Octaplas. LG – Usage in Pediatrics Camazine BS et al. Pediatric Critical Care Med 2017 q All critically ill children admitted to PICU were included if they received at least one plasma unit during 1 week q 419 patients enrolled (62 received octaplas) q Indications: - Critical bleeding - Minor bleeding (surgery and non-surgery) - Planned surgery - High risk of postoperative bleeding - Hypovolemia, abnormal coagulation tests, factor or component replacement Conclusion of the authors Octaplas use in critically ill children may be associated with improved survival. ICU mortality was lower with octaplas vs FFP, 14. 5% versus 29. 1%%, respectively. Octapharma 22

Octaplas. LG – Usage in Pediatrics q Familial hypercholesterolaemia (FH) is the result of a mutation of the Lipoprotein (LDL) Receptor-Gen increased LDL plasma level q Increased LDL increased risk for cardiovascular-disease and an early death q FH prevalence in Denmark 1: 137* q Treatment: Patient with 25 kg BW LDL-apheresis (dialysis) Patient with 25 kg BW plasmapheresis + 5% Albumin (vene access!) Removal of immunoglobulines (Immunosuppression) and plasma proteins *Benn M et al. J Clin Endocrinol Metab, November 2012, 97(11): 3956– 3964 Octapharma 23

Octaplas. LG – Usage in Pediatrics q Case: 4 years old child with an LDL-cholesterol level of 24 mmol/L (Ref < 4 mmol/L) q Treatment: Plasmapheresis every second week between plasmapheresis total cholesterol 15 mmol/L, LDL 14 mmol/L q Plasmapheresis was reduced to weekly to receive an LDL-concentration of <10 mmol/L between the treatments Octapharma 24

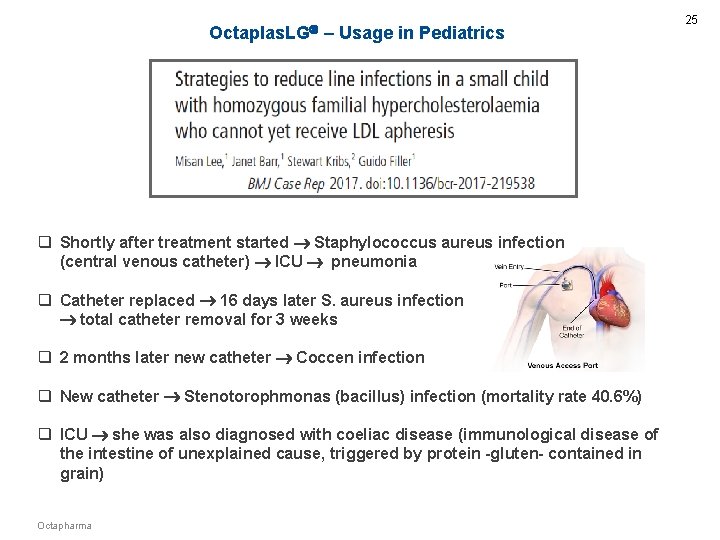
Octaplas. LG – Usage in Pediatrics q Shortly after treatment started Staphylococcus aureus infection (central venous catheter) ICU pneumonia q Catheter replaced 16 days later S. aureus infection total catheter removal for 3 weeks q 2 months later new catheter Coccen infection q New catheter Stenotorophmonas (bacillus) infection (mortality rate 40. 6%) q ICU she was also diagnosed with coeliac disease (immunological disease of the intestine of unexplained cause, triggered by protein -gluten- contained in grain) Octapharma 25

Octaplas. LG – Usage in Pediatrics q Within this 7 months she had: 50 hospital visits 6 surgeries 7 visits to the emergency room 24 plasmapheresis treatments q Treatment changed to: - Her central venous catheter was changed to an implanted port - At the end of each plasmapheresis she received 2 bags of octaplas (Ig. G 8. 1 g/L) to compensate the loss of immunoglobulines q Results: - Her serum Ig. G level increased from 2. 1 to 4. 2 g/L (normal 4. 5– 11. 5 g/L) - Also 9 months after the start of the new treatment she had no infection Octapharma 26

27 Octaplas. LG – Usage in Pediatrics Mohamed ZU, et al. Liver Transplant 2014; 20(S 1): 303 135 pediatrics 7 children Octapharma

Guidelines 28 q Scandinavian guidelines – “The massively bleeding patient” - Scandinavian Journal of Surgery 97: 15– 36, 2008 q Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic Microangiopathies - BJH 2012 q Guideline for the diagnosis and management of the rare coagulation disorders – BJH 2014 q Trends in the diagnosis and management of TTP: European Perspective Transfusion and Apheresis Science 2014 q TRALI Risk Mitigation for Plasma and Whole Blood for Allogeneic Transfusion AABB 2014 q Recommendations concerning products licensed for the treatment of hemophilia and other bleeding orders – Medical and Scientific Advisory Council (MASAC) 2014 q Guidelines on transfusion for fetuses, neonates and older children – BJH 2016 Octapharma

29 Octapla. LG - Product overview Octapharma

Octaplas. LG – Appearance q Frozen pooled plasma q Licensed for the same indications as FFP q Handled in the same way as FFP q Blood-group specific (A, B, 0 or AB) q Available in 200 ml bags q Stored for 4 years at - 18 °C q Post-thaw storage is 24 h at +2 -8°C or 8 h at room temperature at +20 - 25°C q Bags are stored in the Blood Bank Octapharma 30

andrea. neisser-svae@octapharma. com Octapharma
 Fresh frozen plasma transfusion time
Fresh frozen plasma transfusion time Febrile nonhemolytic transfusion reaction
Febrile nonhemolytic transfusion reaction Fresh frozen plasma contents
Fresh frozen plasma contents Platelets transfusion indication
Platelets transfusion indication Cryoprecipitate contents
Cryoprecipitate contents Trali symptoms
Trali symptoms Intimate language register
Intimate language register A food handler can cool a stockpot of clam chowder by
A food handler can cool a stockpot of clam chowder by Frozen slushy liquid time fences
Frozen slushy liquid time fences Frozen section
Frozen section Swot analysis of frozen food business
Swot analysis of frozen food business Frozen foods history
Frozen foods history Frozen quiz
Frozen quiz Diagnosis fisioterapi
Diagnosis fisioterapi Cuff arthropathie
Cuff arthropathie Tes khusus frozen shoulder
Tes khusus frozen shoulder Programma il futuro frozen
Programma il futuro frozen Sphinx welding
Sphinx welding Frozen dew is called
Frozen dew is called Mjukglassmaskiner
Mjukglassmaskiner For the first time in forever piano
For the first time in forever piano Figurative language in let it go frozen
Figurative language in let it go frozen Section cutting procedure
Section cutting procedure Planck's equation for freezing time
Planck's equation for freezing time Dairy foods list
Dairy foods list Lumps of frozen gas and rock
Lumps of frozen gas and rock Niagara falls frozen 1911
Niagara falls frozen 1911 How does diction influence mood
How does diction influence mood Nss frozen food
Nss frozen food Frozen seafood factory
Frozen seafood factory Sam's club eggo waffles
Sam's club eggo waffles Icidh adalah
Icidh adalah