NRG OncologyAlliance LU 005 Limited Stage Small Cell






- Slides: 13

NRG Oncology/Alliance LU 005 Limited Stage Small Cell Lung Cancer: A Phase II/III Trial of Chemoradiation +/- Atezolizumab Kristin Higgins, MD NRG Oncology Semi-Annual Meeting July 17, 2020 @NRGOnc NRG Oncology

Disclosures • Refle. Xion Medical Funded Research • Astra Zeneca Advisory Board and Consultant

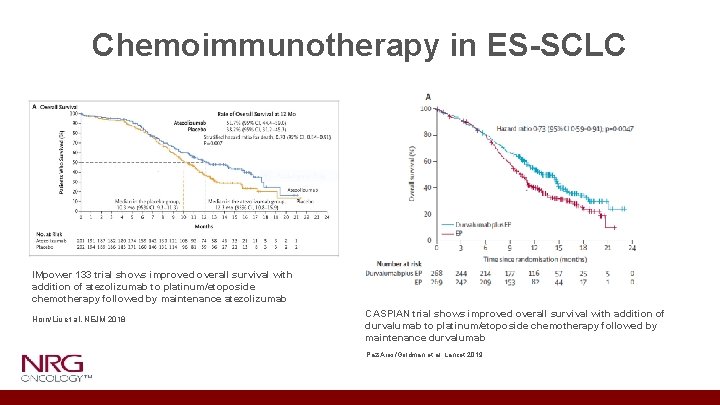
Chemoimmunotherapy in ES-SCLC IMpower 133 trial shows improved overall survival with addition of atezolizumab to platinum/etoposide chemotherapy followed by maintenance atezolizumab Horn/Liu et al, NEJM 2018 CASPIAN trial shows improved overall survival with addition of durvalumab to platinum/etoposide chemotherapy followed by maintenance durvalumab Paz-Ares/Goldman et al, Lancet 2019

• Significance of this approval is substantial given no new agents have demonstrated survival gains in SCLC in several decades!! • Platinum/etoposide + atezolizumab or platinum/etoposide + durvalumab is the new standard of care for ES-SCLC

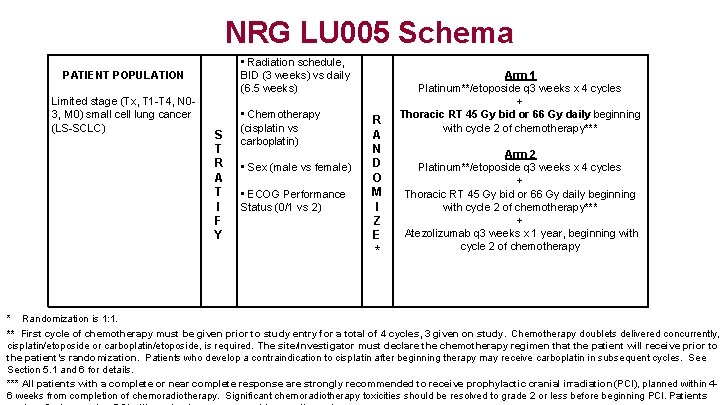
NRG LU 005 Schema • Radiation schedule, BID (3 weeks) vs daily (6. 5 weeks) PATIENT POPULATION Limited stage (Tx, T 1 -T 4, N 03, M 0) small cell lung cancer (LS-SCLC) S T R A T I F Y • Chemotherapy (cisplatin vs carboplatin) • Sex (male vs female) • ECOG Performance Status (0/1 vs 2) R A N D O M I Z E * Arm 1 Platinum**/etoposide q 3 weeks x 4 cycles + Thoracic RT 45 Gy bid or 66 Gy daily beginning with cycle 2 of chemotherapy*** Arm 2 Platinum**/etoposide q 3 weeks x 4 cycles + Thoracic RT 45 Gy bid or 66 Gy daily beginning with cycle 2 of chemotherapy*** + Atezolizumab q 3 weeks x 1 year, beginning with cycle 2 of chemotherapy * Randomization is 1: 1. ** First cycle of chemotherapy must be given prior to study entry for a total of 4 cycles, 3 given on study. Chemotherapy doublets delivered concurrently, cisplatin/etoposide or carboplatin/etoposide, is required. The site/investigator must declare the chemotherapy regimen that the patient will receive prior to the patient’s randomization. Patients who develop a contraindication to cisplatin after beginning therapy may receive carboplatin in subsequent cycles. See Section 5. 1 and 6 for details. *** All patients with a complete or near complete response are strongly recommended to receive prophylactic cranial irradiation (PCI), planned within 46 weeks from completion of chemoradiotherapy. Significant chemoradiotherapy toxicities should be resolved to grade 2 or less before beginning PCI. Patients

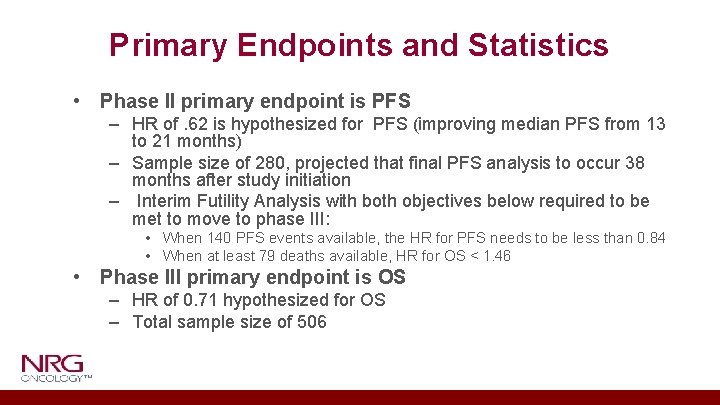
Primary Endpoints and Statistics • Phase II primary endpoint is PFS – HR of. 62 is hypothesized for PFS (improving median PFS from 13 to 21 months) – Sample size of 280, projected that final PFS analysis to occur 38 months after study initiation – Interim Futility Analysis with both objectives below required to be met to move to phase III: • When 140 PFS events available, the HR for PFS needs to be less than 0. 84 • When at least 79 deaths available, HR for OS < 1. 46 • Phase III primary endpoint is OS – HR of 0. 71 hypothesized for OS – Total sample size of 506

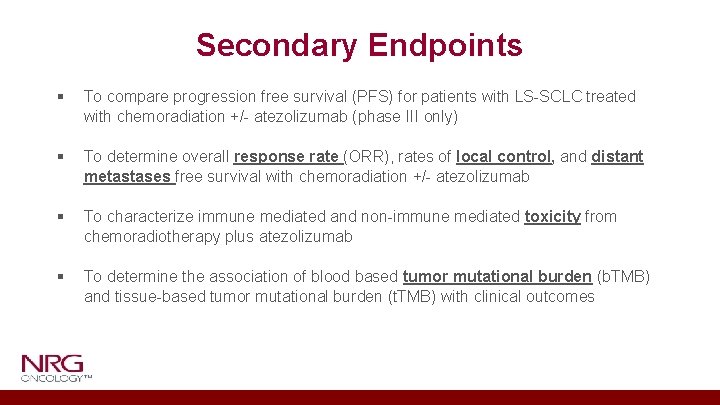
Secondary Endpoints § To compare progression free survival (PFS) for patients with LS-SCLC treated with chemoradiation +/- atezolizumab (phase III only) § To determine overall response rate (ORR), rates of local control, and distant metastases free survival with chemoradiation +/- atezolizumab § To characterize immune mediated and non-immune mediated toxicity from chemoradiotherapy plus atezolizumab § To determine the association of blood based tumor mutational burden (b. TMB) and tissue-based tumor mutational burden (t. TMB) with clinical outcomes

Quality of Life Secondary Endpoints § To compare quality of life, as measured by the FACT-TOI, for patients undergoing chemoradiation +/- atezolizumab § To evaluate the quality-adjusted survival, using scores from the EQ 5 D-5 L, of chemoradiotherapy +/- atezolizumab for patients with LSSCLC § To characterize fatigue, as measured by the PROMIS, following chemoradiation +/- atezolizumab

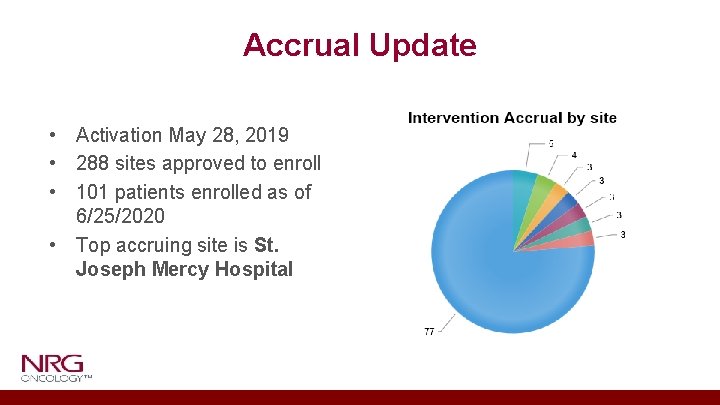
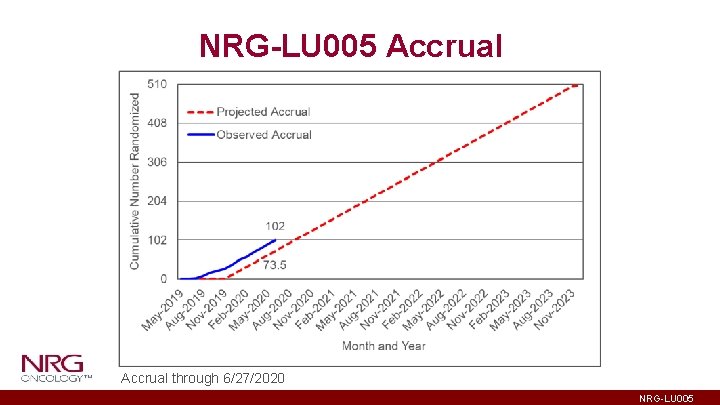
Accrual Update • Activation May 28, 2019 • 288 sites approved to enroll • 101 patients enrolled as of 6/25/2020 • Top accruing site is St. Joseph Mercy Hospital

NRG-LU 005 Accrual through 6/27/2020 NRG-LU 005

Next Amendment – Later this Summer • Will lengthen window for staging PET/CT (60 days from 45) • e. GFR loosened to allow e. GFR of 30 or higher • Clarify language around tissue submission

NRG LU 005 Study Team Kristin A. Higgins, MD; Winship Cancer Institute of Emory University PI/ Rad. Oncology Helen J. Ross, MD; Mayo Clinic Arizona Salma Jabbour, MD; Rutgers Cancer Institute of New Jersey David Kozono, MD, Ph. D; Dana-Farber Cancer Institute Taofeek K. Owonikoko, MD, Ph. D, MSCR; Winship Cancer Institute, Emory University Co. PI/ Med. Oncology Jeffry P. Simko, Ph. D, MD; University of California San Francisco Pathology Timothy D. Solberg, Ph. D; University of California San Francisco Physics Ben Movas, MD; Henry Ford Health System Canhua Xiao, Ph. D RN; Yale University Quality of Life James Welsh, MD; MD Anderson Cancer Center Translational Science/Co. Chair Terence Williams, MD, Ph. D; The Ohio State University Chen Hu, Ph. D; NRG Oncology Xiaofei Wang, Ph. D; Duke Biostatistics & Bioinformatics Rad. Oncology Med. Oncology Quality of Life Statistics

Acknowledgements NRG LU 005 Study Team Genentech partnership This project is supported by grants U 10 CA 180868 (NRG Oncology Operations) from the National Cancer Institute (NCI); Clinical. Trials. gov/NCT 03811002, and Genetech @NRGOnc NRG Oncology