NRG Oncology Town Hall NRG Virtual Summer Meeting














- Slides: 22

NRG Oncology Town Hall NRG Virtual Summer Meeting 2020 10 -11 am ET @NRGOnc NRG Oncology

Ensuring Cancer Research Progress Amid a Global Pandemic Norman E. Sharpless, M. D. NRG Oncology Virtual Meeting July 17, 2020 @NCIDirector @The. NCI

Today’s presentation § Budget outlook § NCI response to COVID-19 § Clinical Research Updates § Addressing racial inequity in cancer research § Q&A 3

NCI Appropriations FY 2015 – 2020 (in millions) $5, 689 $300 $6 000 $5 000 [VALUE] 21 st Century Cures Act - orange Childhood Cancer Initiative - green $5, 965 $300 $6, 144 $400 [VALUE] $6, 494 $6, 440 [VALUE] $6, 195 $50 + $306 M $4 000 COVID-19 serology (April 2020) $3 000 $6 249 $6, 249 $195 $50 + $414 M emergency funding $2 000 $1 000 $0 FY 2015 FY 2016 FY 2017 FY 2018 FY 2019 FY 2020 FY 2021 House Mark 4

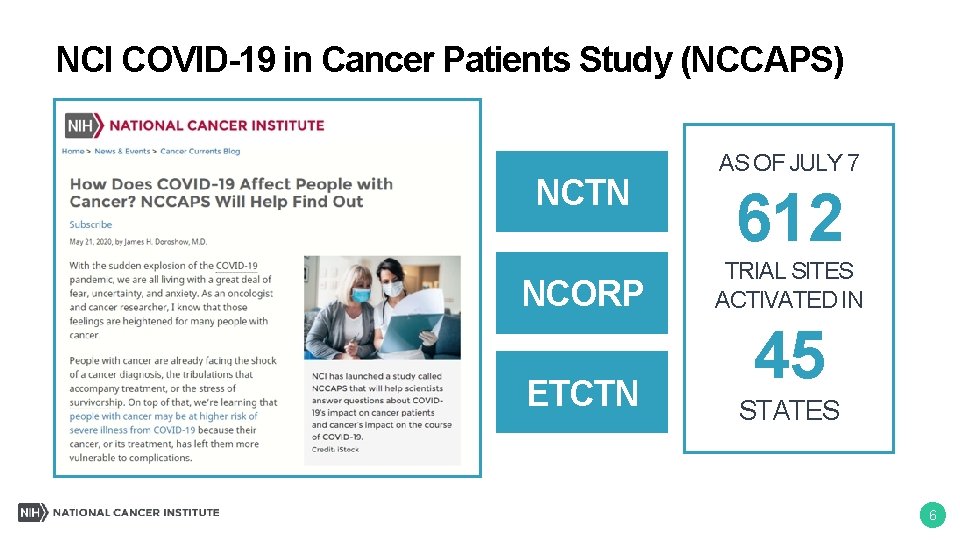
NCI Response to COVID-19 • SARS-Co. V-2 serology research • NCI COVID-19 in Cancer Patients Study (NCCAPS) • Guidance for cancer clinical trials • Flexibilities and opportunities for grantees • Genomic studies of COVID-19 outcomes • Trials of immunomodulatory agents for severe COVID -19 pneumonia cancer. gov/coronavirus-researchers 5

NCI COVID-19 in Cancer Patients Study (NCCAPS) NCTN NCORP ETCTN AS OF JULY 7 612 TRIAL SITES ACTIVATED IN 45 STATES 6

Flexibilities to support grantees during the pandemic Extending deadlines for applications Allowing institutions to use NCI grant funds to maintain salaries and stipends Extending project timelines and reporting requirements Extending eligibility periods for early-stage investigators and trainees Carryover for institutional training grants (T 35, T 32, K 12) with prior approval 7

Emergency Support for Postdoctoral Fellows during COVID-19 Notice of Special Interest (NOSI): NOT-CA-20 -082 Administrative supplements to cover salary for a postdoctoral fellow whose stipend support from a non-profit funder has been lost because of the COVID-19 global pandemic. 8

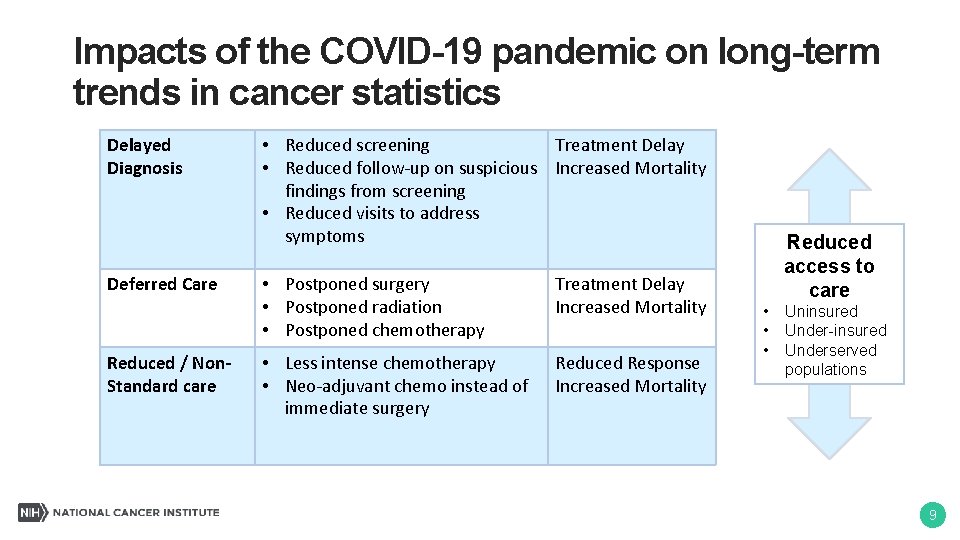
Impacts of the COVID-19 pandemic on long-term trends in cancer statistics Delayed Diagnosis Deferred Care Reduced / Non. Standard care • Reduced screening Treatment Delay • Reduced follow-up on suspicious Increased Mortality findings from screening • Reduced visits to address symptoms • Postponed surgery • Postponed radiation • Postponed chemotherapy Treatment Delay Increased Mortality • Less intense chemotherapy • Neo-adjuvant chemo instead of immediate surgery Reduced Response Increased Mortality Reduced access to care • • • Uninsured Under-insured Underserved populations 9

19 June 2020 10

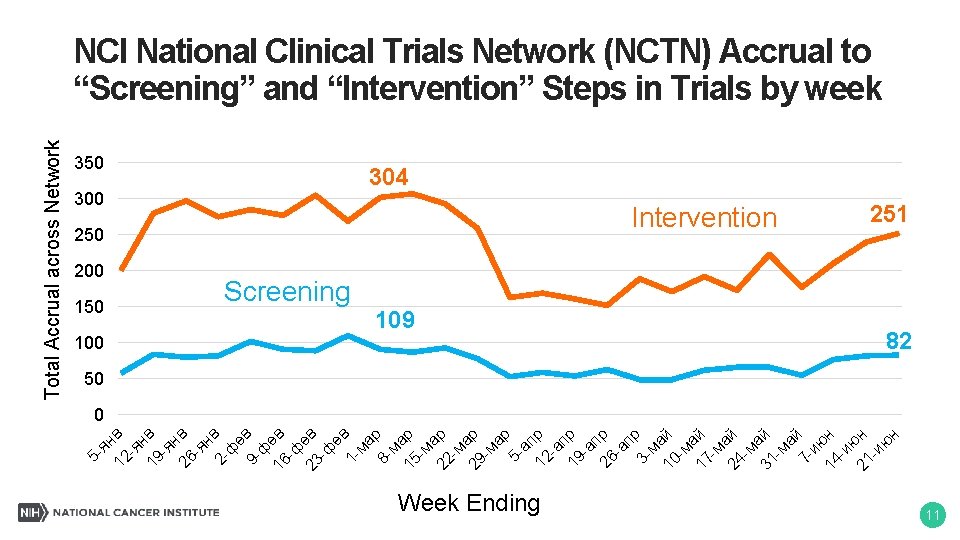
нв 200 150 е 9 - в ф 16 ев -ф 23 ев -ф ев 1 ма р 8 ма 15 р -м а 22 р -м а 29 р -м ар 5 ап 12 р -а п 19 р -а п 26 р -а пр 3 ма 10 й -м а 17 й -м а 24 й -м а 31 й -м ай 7 ию 14 н -и ю 21 н -и ю н ф 2 - нв -я 26 в нв -я 19 -я 12 ян 5 - Total Accrual across Network NCI National Clinical Trials Network (NCTN) Accrual to “Screening” and “Intervention” Steps in Trials by week 350 304 250 Intervention Screening 100 109 Week Ending 251 82 50 0 11

Adapting clinical trials during the pandemic • Some study agents may be shipped to patients • Patient care may be transferred to other study sites • Some services can be provided by local providers, with oversight by responsible investigator • “Remote” informed consent (telephone + signature) • Minor deviations: study visits by telemedicine rather than inperson; delayed study visits; delayed lab or imaging tests; minimal treatment delays; biospecimen collections 12

During the COVID-19 pandemic, has your site used the following modified clinical trials processes for NCTN trials? Average Usefulness Yes No 1 - not at all useful to 5 very useful Worked with local healthcare providers to provide continuity of care for patients on NCTN trials 52 31 3. 9 (n=63) Used virtual (telehealth / telephone) study visits 85 5 4. 6 (n=88) Shipped oral IND agents directly to patients enrolled 53 26 4. 5 (n=63) Used remote informed consent to enroll patients 51 35 4. 2 (n=64) Underwent a remote audit by an NCTN group 20 66 3. 6 (n=29) 13

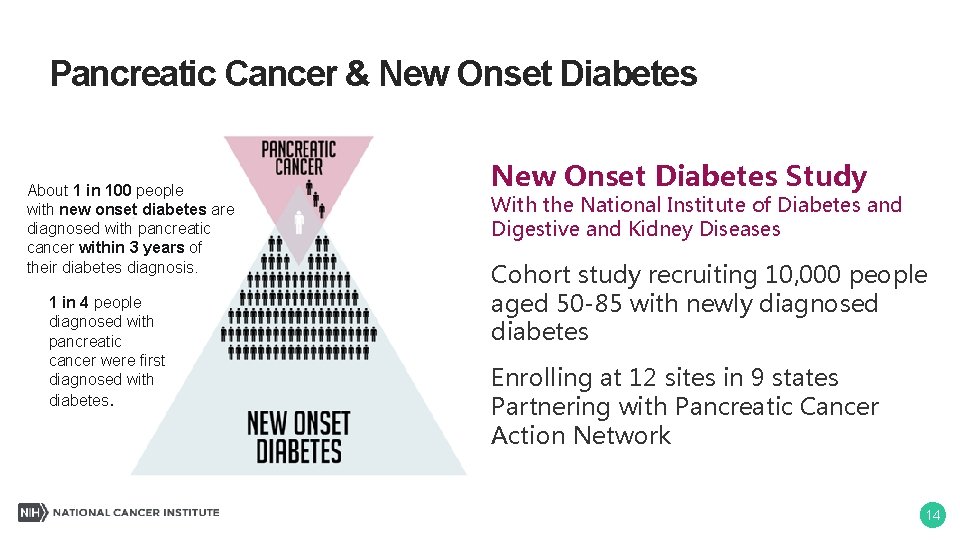
Pancreatic Cancer & New Onset Diabetes About 1 in 100 people with new onset diabetes are diagnosed with pancreatic cancer within 3 years of their diabetes diagnosis. 1 in 4 people diagnosed with pancreatic cancer were first diagnosed with diabetes. New Onset Diabetes Study With the National Institute of Diabetes and Digestive and Kidney Diseases Cohort study recruiting 10, 000 people aged 50 -85 with newly diagnosed diabetes Enrolling at 12 sites in 9 states Partnering with Pancreatic Cancer Action Network 14

Pembrolizumab for TMB-H tumors 15

Improvement in Patient-Reported Outcomes With Intensity. Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study J Clin Oncol. 2020 May 20; 38(15): 1685 -1692. Epub 2020 Feb 19. 16

NCI Efforts to Promote Racial Equity Addressing Cancer Health Disparities Promoting Workforce Diversity Creating an Inclusive Culture at NCI 17

Minority accrual to clinical trials 90% Accrual to NCTN and NCORP trials (and predecessor programs) Phases I – III, 1999 – 2019 80% 70% Majority 60% 50% 40% Minority 30% Black or African American 20% 10% 0% 1999 -2001 2002 -2004 2005 -2007 2008 -2010 2011 -2013 2014 -2016 2017 -2019 18

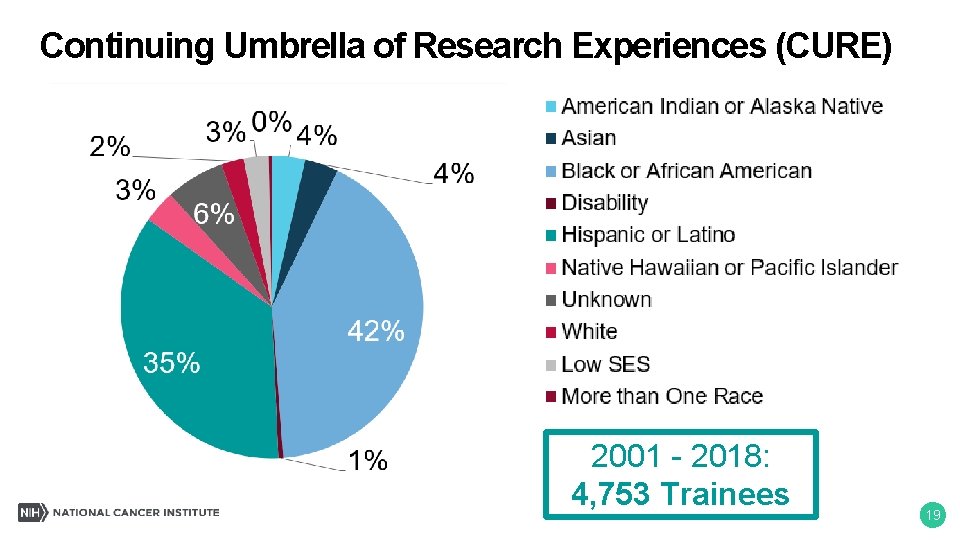
Continuing Umbrella of Research Experiences (CURE) 2001 - 2018: 4, 753 Trainees 19

The Cancer Letter, June 5, 2020 20

www. cancer. gov Thank you! www. cancer. gov/espanol 1 -800 -4 -CANCER NCIinfo@nih. gov @NCIDirector @The. NCI

Question and Answer session Panel view Wally Curran Norm Wolmark Robert Mannel Deb Bruner Joan Walker Ned Sharpless @NRGOnc NRG Oncology
 Nrg virtual meeting
Nrg virtual meeting Nrg oncology meeting 2019
Nrg oncology meeting 2019 Nrg oncology meeting 2019
Nrg oncology meeting 2019 Town b is 380 km due south of town a
Town b is 380 km due south of town a Sophiatown act 1 scene 1 summary
Sophiatown act 1 scene 1 summary East rockaway town hall
East rockaway town hall Chapin town hall
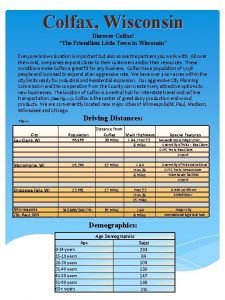
Chapin town hall Colfax town hall
Colfax town hall Jwst town hall
Jwst town hall Waverly village hall
Waverly village hall Cohasset dpw
Cohasset dpw St joseph church penfield
St joseph church penfield Peabody town hall
Peabody town hall Henley town hall
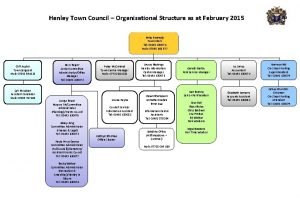
Henley town hall Killingly town hall
Killingly town hall What is meeting and types of meeting
What is meeting and types of meeting Types of meeting
Types of meeting Today meeting or today's meeting
Today meeting or today's meeting Proposal kickoff meeting agenda
Proposal kickoff meeting agenda Virtual meeting etiquette tips
Virtual meeting etiquette tips Joanne chien
Joanne chien Site:slidetodoc.com
Site:slidetodoc.com Has virtual functions and accessible non-virtual destructor
Has virtual functions and accessible non-virtual destructor