Natural History of Picornavirus Colds in Adults 69






























- Slides: 42

Natural History of Picornavirus Colds in Adults • • • 69% self-diagnosed cold within 8 hours Sore throat most common first symptom Rhinorrhea most bothersome symptom Fever uncommon Sleep disturbed 4 days 7 -to 11 -day duration of symptoms – 25% have symptoms for 2 weeks Arruda, et al. J Clin Micro. 1997; 35: 2864 Monto, et al. J Infect Dis. 1987; 156: 43 Gwaltney, et al. JAMA. 1967; 202: 294


Current Management of Colds • Leading reason for physician visits – ~17% of colds result in an office visit • Antibiotics – 30 -50% of visits result in antibiotic prescription – No reduction in symptoms or complications • No treatment for the underlying viral cause Mc. Isaac, et al. J Fam Prac. 1998; 47: 366 Gonzales, et al. JAMA. 1997; 278: 901 Gonzales, et al. Ann Intern Med. 2001; 134: 479 Rosenstein, et al. Pediatrics. 1998; 101: 181

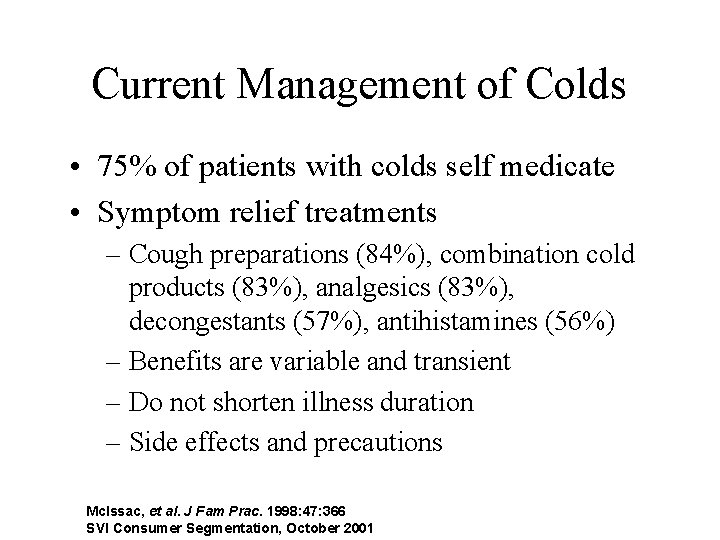
Current Management of Colds • 75% of patients with colds self medicate • Symptom relief treatments – Cough preparations (84%), combination cold products (83%), analgesics (83%), decongestants (57%), antihistamines (56%) – Benefits are variable and transient – Do not shorten illness duration – Side effects and precautions Mc. Issac, et al. J Fam Prac. 1998: 47: 366 SVI Consumer Segmentation, October 2001


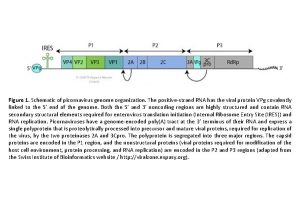
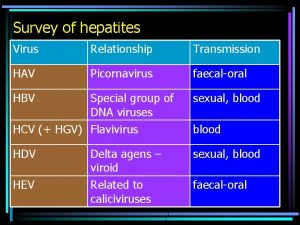
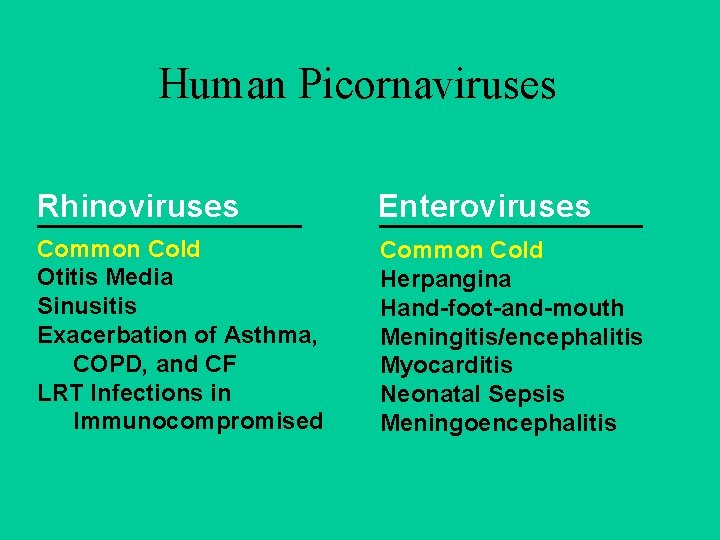
Human Picornaviruses Rhinoviruses Enteroviruses Common Cold Otitis Media Sinusitis Exacerbation of Asthma, COPD, and CF LRT Infections in Immunocompromised Common Cold Herpangina Hand-foot-and-mouth Meningitis/encephalitis Myocarditis Neonatal Sepsis Meningoencephalitis

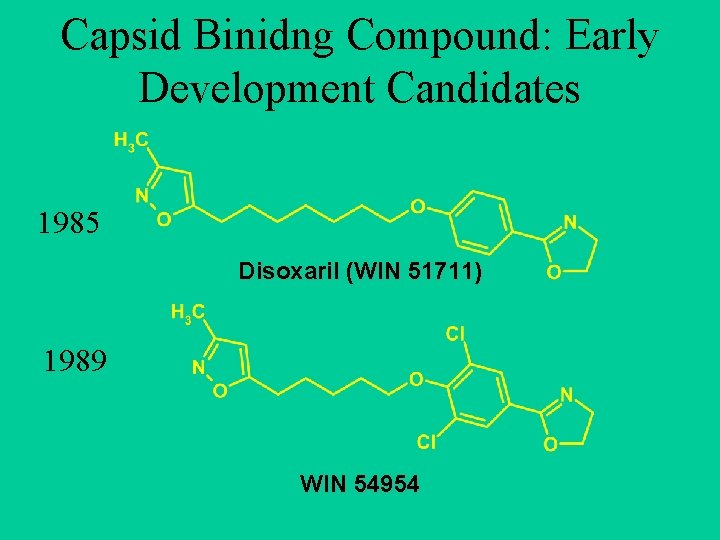
Capsid Binidng Compound: Early Development Candidates 1985 Disoxaril (WIN 51711) 1989 WIN 54954

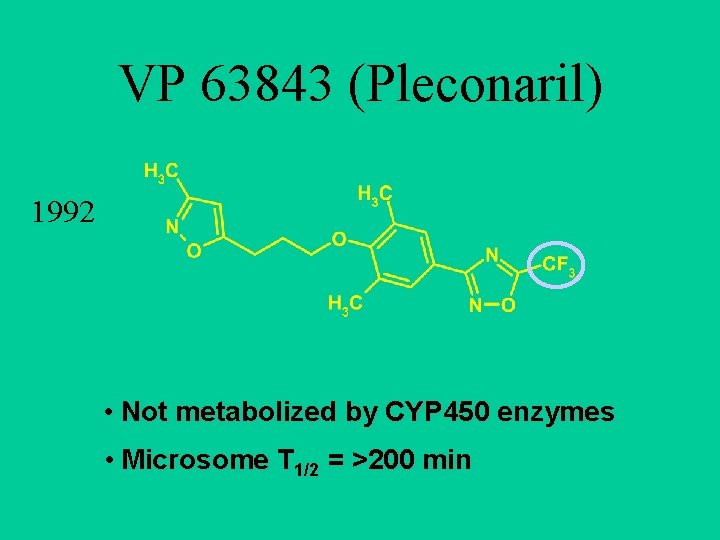
VP 63843 (Pleconaril) 1992 • Not metabolized by CYP 450 enzymes • Microsome T 1/2 = >200 min


Protection by Pleconaril of Adult Mice Infected With CVB 3 Pevear et al Antimicrob Agents Chemother, 1999.

Structural Studies of Anti-rhinovirus Agents 1985 - 2002 Purdue University Eddy Arnold Sungsoo Kim John Badger S. Krishnaswamy Michael Chapman Ming Luo Andrea Hadfield Jodi Muckelbauer Kyung Kim Marcos Olivera Viro. Pharma(Stirling Winthrop) Guy Diana Frank Dutko Jim Groarke Mark Mc. Kinlay Dan Pevear Alan Simpson Tom Smith Gerd Vriend Rui Zhao Ying Zhang University of Wisconsin Beverly Heinz Wai-Ming Lee Roland Rueckert Debbie Shepard Wensheng Wang







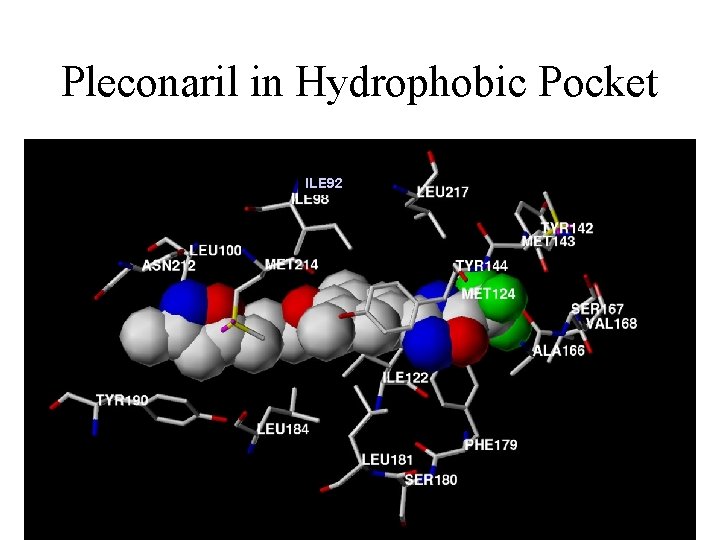
Pleconaril in Hydrophobic Pocket ILE 92



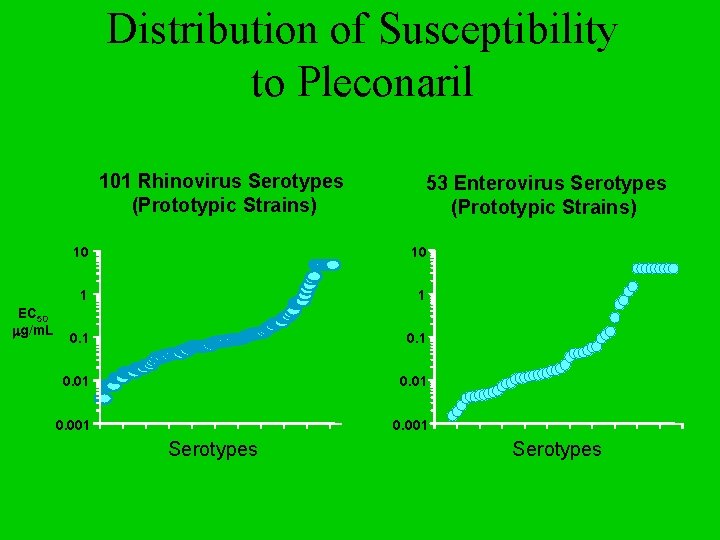
Distribution of Susceptibility to Pleconaril 101 Rhinovirus Serotypes (Prototypic Strains) EC 50 mg/m. L 53 Enterovirus Serotypes (Prototypic Strains) 10 10 1 1 0. 01 0. 001 Serotypes





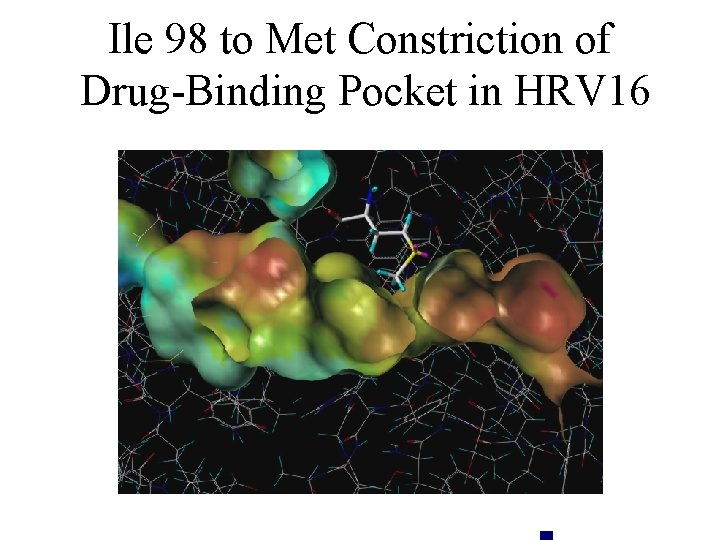
Ile 98 to Met Constriction of Drug-Binding Pocket in HRV 16

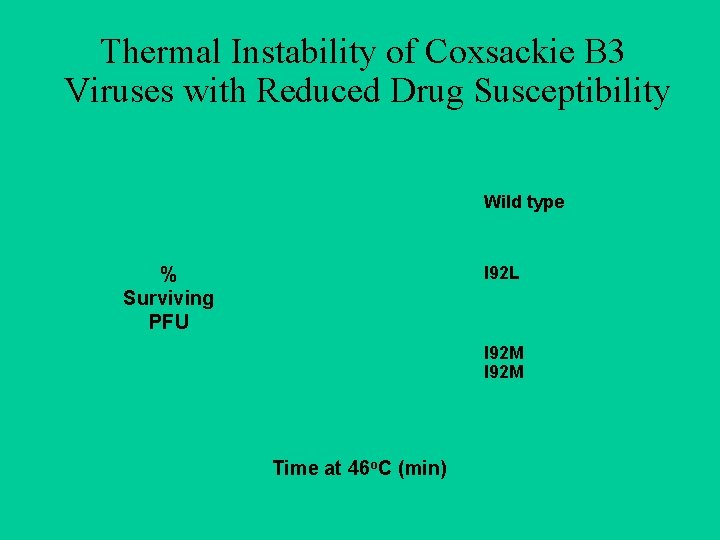
Thermal Instability of Coxsackie B 3 Viruses with Reduced Drug Susceptibility Wild type % Surviving PFU I 92 L I 92 M Time at 46 o. C (min)

First Phase 3 Human Clinical Trials with Pleconaril

Coxsackie Respiratory Infection Mucus Production P = 0. 016

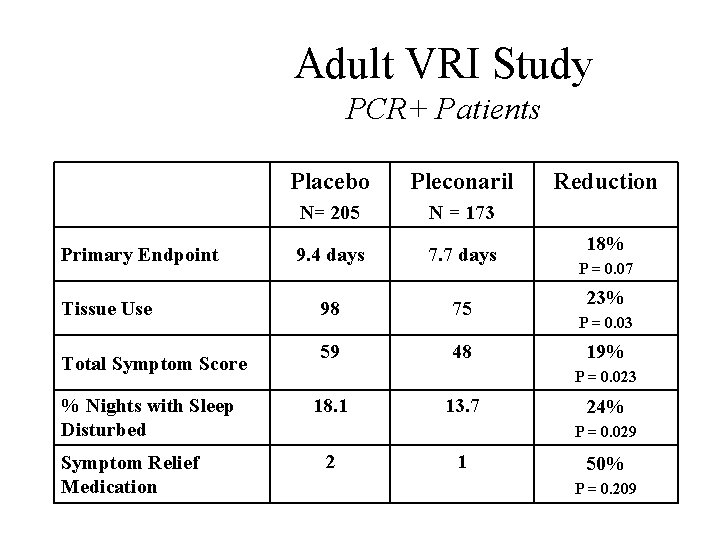
Adult VRI Study PCR+ Patients Primary Endpoint Tissue Use Total Symptom Score % Nights with Sleep Disturbed Symptom Relief Medication Placebo Pleconaril N= 205 N = 173 9. 4 days 7. 7 days 98 75 59 48 Reduction 18% P = 0. 07 23% P = 0. 03 19% P = 0. 023 18. 1 13. 7 24% P = 0. 029 2 1 50% P = 0. 209

Second Phase 3 Human Clinical Trials with Pleconaril

h a Phase 3 design s e • Two randomized, placebo-controlled trials of identical design • 2096 patients randomized – Protocol 043: 1052 patients – Protocol 044: 1044 patients • 197 centers across the US and Canada • Enrollment from August – November 2000

Entry Criteria • • Otherwise healthy subjects ≥ 18 years old Answer ‘Yes’ to “Do you have a cold today? ” Moderate or severe rhinorrhea At least one other respiratory symptom – nasal congestion, cough, sore throat • Symptom duration ≤ 24 hrs • Exclusions – active allergic rhinitis or asthma – fever ≥ 100ºF

Patient Self-Assessments (Days 1 -18) • Rhinorrhea, nasal congestion, cough, sore throat, malaise, myalgia: absent, mild, moderate, or severe, twice daily • Presence or absence of cold twice daily • Tissue counts once daily • Sleep disturbance once daily • Impairment of normal activity level once daily • Concomitant use of cold symptom relief medications

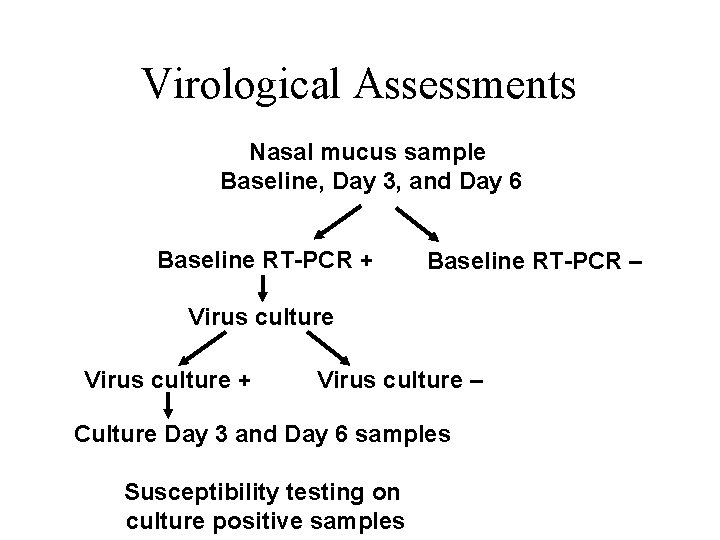
Virological Assessments Nasal mucus sample Baseline, Day 3, and Day 6 Baseline RT-PCR + Baseline RT-PCR – Virus culture + Virus culture – Culture Day 3 and Day 6 samples Susceptibility testing on culture positive samples

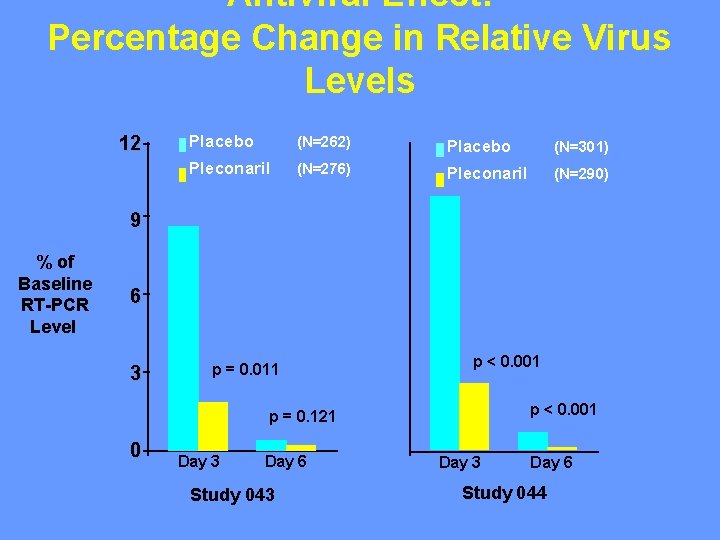
Antiviral Effect: Percentage Change in Relative Virus Levels 12 Placebo (N=262) Placebo (N=301) Pleconaril (N=276) Pleconaril (N=290) 9 % of Baseline RT-PCR Level 6 3 p = 0. 011 p < 0. 001 p = 0. 121 0 Day 3 Day 6 Study 043 Day 6 Study 044

Safety Conclusions: 5 Day Treatment • Most common adverse events were headache and GI symptoms • No clinically significant effects on laboratory safety parameters • Increased menstrual disorders in OC users; 3. 5% with pleconaril treatment dose • No evidence of increased incidence of pregnancy in women taking pleconaril • Safety profile supports empiric treatment of colds

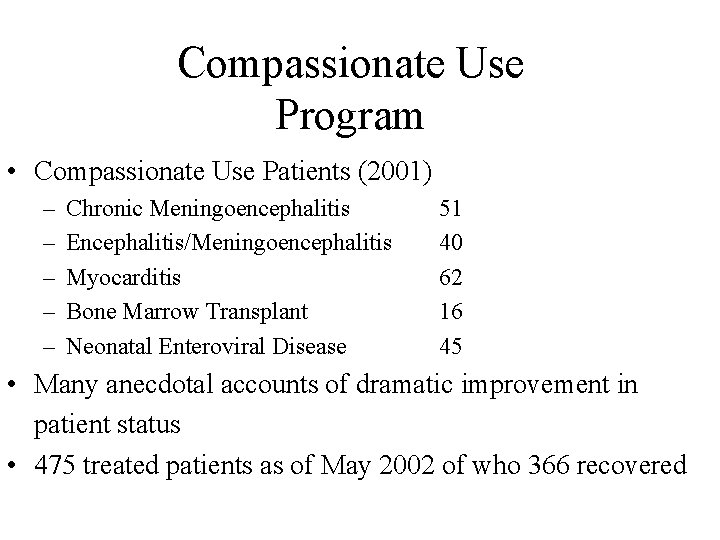
Compassionate Use Program • Compassionate Use Patients (2001) – – – Chronic Meningoencephalitis Encephalitis/Meningoencephalitis Myocarditis Bone Marrow Transplant Neonatal Enteroviral Disease 51 40 62 16 45 • Many anecdotal accounts of dramatic improvement in patient status • 475 treated patients as of May 2002 of who 366 recovered

Conclusions • Pleconaril is the first antiviral drug to treat the predominant cause of the common cold • Pleconaril reduces the duration and severity of picornavirus colds • Safety profile supports empiric treatment

FDA committee decision th March 19 2002 1. Potential of producing virulent viral strains too great relative to benefits gained 2. Problem of women on birth control drugs: warning messages are thought to be ineffective 3. Benefits of an anti-common cold drug too small relative to possible risks of side effects. 4. Concern over inappropriate use with the possibility of generating virulent strains 5. Committee voted 15 to 0 against licensing of drug.


There could be a place for you at Purdue University!
 Picornavirus adalah
Picornavirus adalah Picornavirus familia
Picornavirus familia Natural hazards vs natural disasters
Natural hazards vs natural disasters Natural capital and natural income
Natural capital and natural income The plastic pink flamingo a natural history
The plastic pink flamingo a natural history Natural history of disease adalah
Natural history of disease adalah Natural history of disease
Natural history of disease Natural history of disease diagram
Natural history of disease diagram Natural history of disease is best studied by
Natural history of disease is best studied by National museum british
National museum british Natural history and spectrum of disease
Natural history and spectrum of disease American musuem of natural history
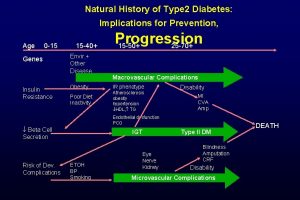
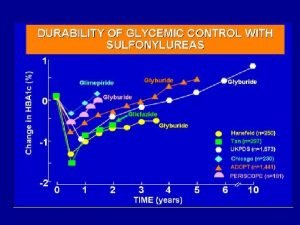
American musuem of natural history Natural history of type 2 diabetes
Natural history of type 2 diabetes Tasha introduction
Tasha introduction Natural history of type 2 diabetes
Natural history of type 2 diabetes Spectrum of disease
Spectrum of disease Selective mutism in adults
Selective mutism in adults Normal vital signs for adults
Normal vital signs for adults Respiratory rate normal
Respiratory rate normal Bluegrass billbugs overwinter as adults
Bluegrass billbugs overwinter as adults Love languages adults
Love languages adults Sites of pulse
Sites of pulse Examples of problem-solving scenarios
Examples of problem-solving scenarios Amoebiasis stool color for adults
Amoebiasis stool color for adults Self-advocacy worksheets for adults
Self-advocacy worksheets for adults Pyloromyotomy
Pyloromyotomy Odd diagnostic criteria
Odd diagnostic criteria Nvld
Nvld Jaundice grading in adults
Jaundice grading in adults Token economy in psychology
Token economy in psychology Mental health and older adults
Mental health and older adults Sepsis infection pictures
Sepsis infection pictures Flask shaped
Flask shaped Hepatitis b vaccine schedule for adults
Hepatitis b vaccine schedule for adults Scenarios of unhealthy relationships
Scenarios of unhealthy relationships General ability measure for adults
General ability measure for adults Riddles vegetables
Riddles vegetables How many molars do adults have
How many molars do adults have Placement of aed pads on adults
Placement of aed pads on adults Beth conover
Beth conover Hpv vaccine schedule adults
Hpv vaccine schedule adults Idea definition of emotional disturbance
Idea definition of emotional disturbance Mental health and older adults
Mental health and older adults