Molecular Mechanics force fields minimization Force Fields good
- Slides: 16

Molecular Mechanics • force fields • minimization

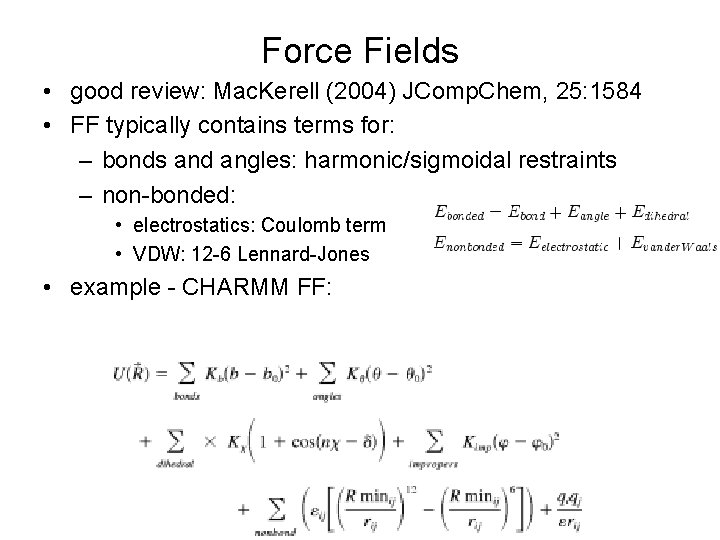
Force Fields • good review: Mac. Kerell (2004) JComp. Chem, 25: 1584 • FF typically contains terms for: – bonds and angles: harmonic/sigmoidal restraints – non-bonded: • electrostatics: Coulomb term • VDW: 12 -6 Lennard-Jones • example - CHARMM FF:

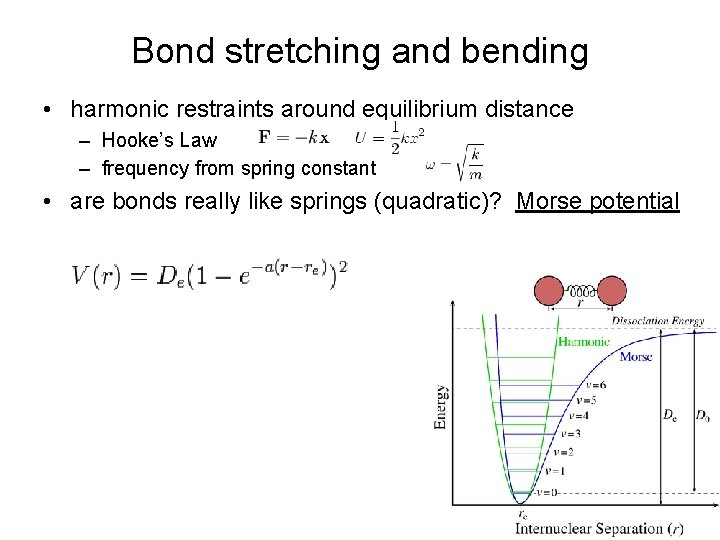
Bond stretching and bending • harmonic restraints around equilibrium distance – Hooke’s Law – frequency from spring constant • are bonds really like springs (quadratic)? Morse potential

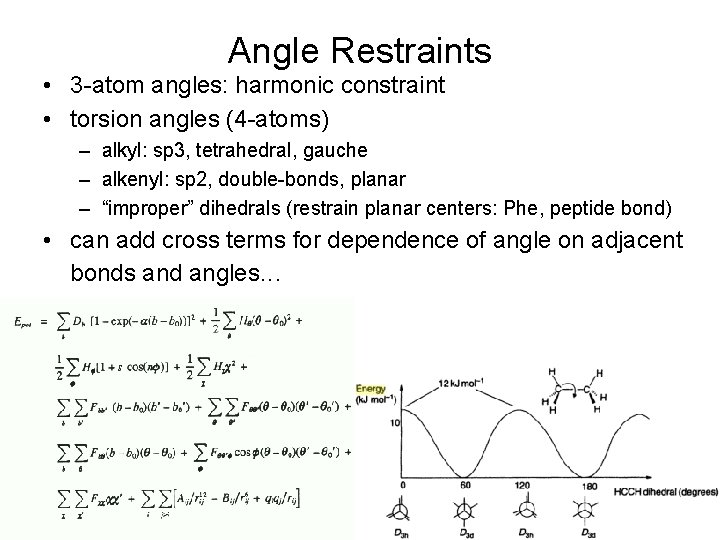
Angle Restraints • 3 -atom angles: harmonic constraint • torsion angles (4 -atoms) – alkyl: sp 3, tetrahedral, gauche – alkenyl: sp 2, double-bonds, planar – “improper” dihedrals (restrain planar centers: Phe, peptide bond) • can add cross terms for dependence of angle on adjacent bonds and angles. . .

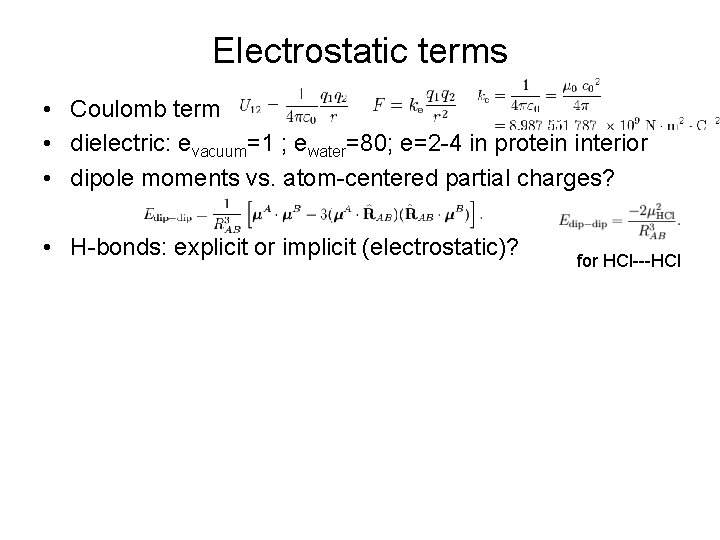
Electrostatic terms • Coulomb term • dielectric: evacuum=1 ; ewater=80; e=2 -4 in protein interior • dipole moments vs. atom-centered partial charges? • H-bonds: explicit or implicit (electrostatic)? for HCl---HCl

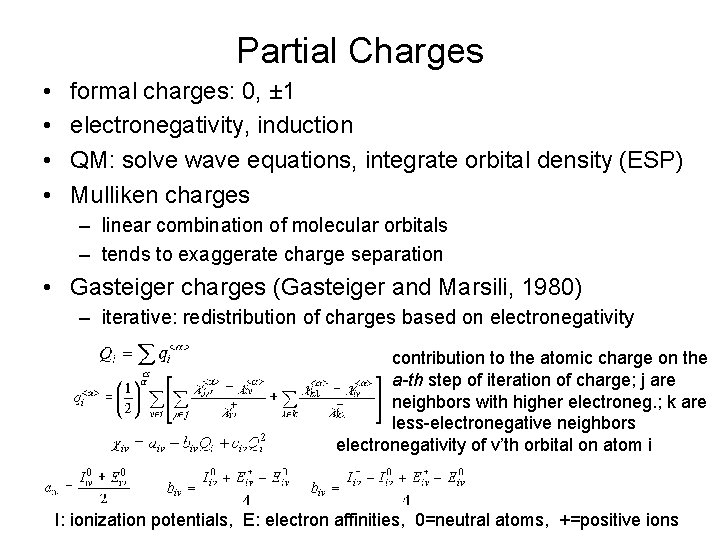
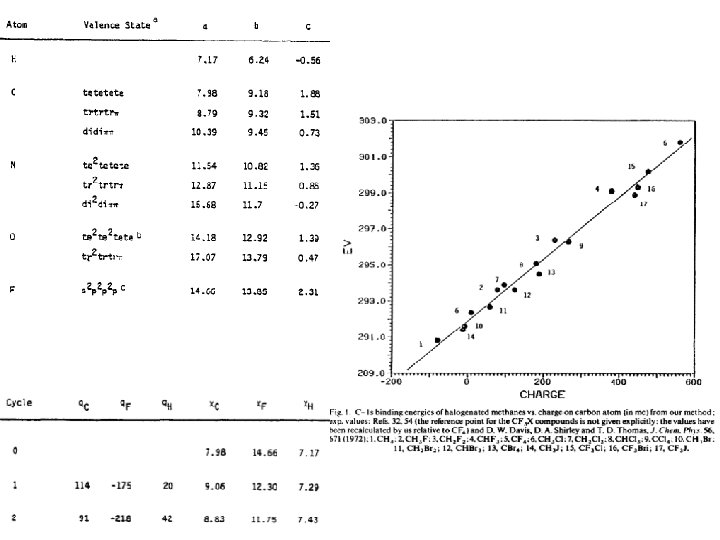
Partial Charges • • formal charges: 0, ± 1 electronegativity, induction QM: solve wave equations, integrate orbital density (ESP) Mulliken charges – linear combination of molecular orbitals – tends to exaggerate charge separation • Gasteiger charges (Gasteiger and Marsili, 1980) – iterative: redistribution of charges based on electronegativity contribution to the atomic charge on the a-th step of iteration of charge; j are neighbors with higher electroneg. ; k are less-electronegative neighbors electronegativity of v’th orbital on atom i I: ionization potentials, E: electron affinities, 0=neutral atoms, +=positive ions


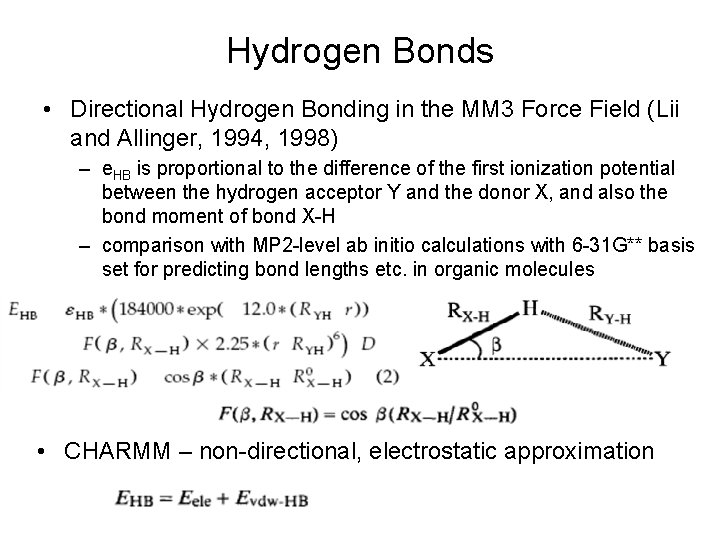
Hydrogen Bonds • Directional Hydrogen Bonding in the MM 3 Force Field (Lii and Allinger, 1994, 1998) – e. HB is proportional to the difference of the first ionization potential between the hydrogen acceptor Y and the donor X, and also the bond moment of bond X-H – comparison with MP 2 -level ab initio calculations with 6 -31 G** basis set for predicting bond lengths etc. in organic molecules • CHARMM – non-directional, electrostatic approximation

Common FF Parameterizations • all-atom vs. united atom (only polar H’s) • parameterize on small organic molecules – acetamide, cyclohexane. . . – predict vibrational spectra, melting temperatures, conformational/solvation energies. . . • • • AMBER (Cornell 1995) OPLS (Jorgensen) Optimized Potential for Liquid Simulations MM 3 (Allinger et al. , 1989) MMFF 94 (Merck) (Halgren, 1996) Charmm (Karplus) NAMD, Gromos, ECEPP, CFF. . .

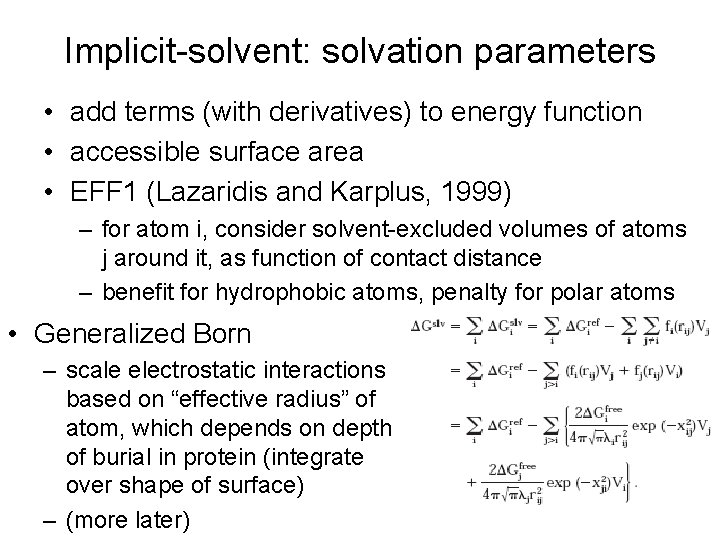
Implicit-solvent: solvation parameters • add terms (with derivatives) to energy function • accessible surface area • EFF 1 (Lazaridis and Karplus, 1999) – for atom i, consider solvent-excluded volumes of atoms j around it, as function of contact distance – benefit for hydrophobic atoms, penalty for polar atoms • Generalized Born – scale electrostatic interactions based on “effective radius” of atom, which depends on depth of burial in protein (integrate over shape of surface) – (more later)

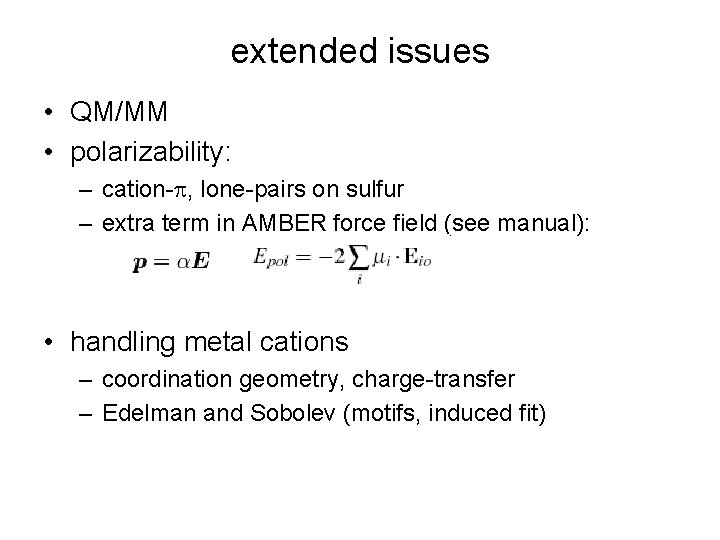
extended issues • QM/MM • polarizability: – cation-p, lone-pairs on sulfur – extra term in AMBER force field (see manual): • handling metal cations – coordination geometry, charge-transfer – Edelman and Sobolev (motifs, induced fit)

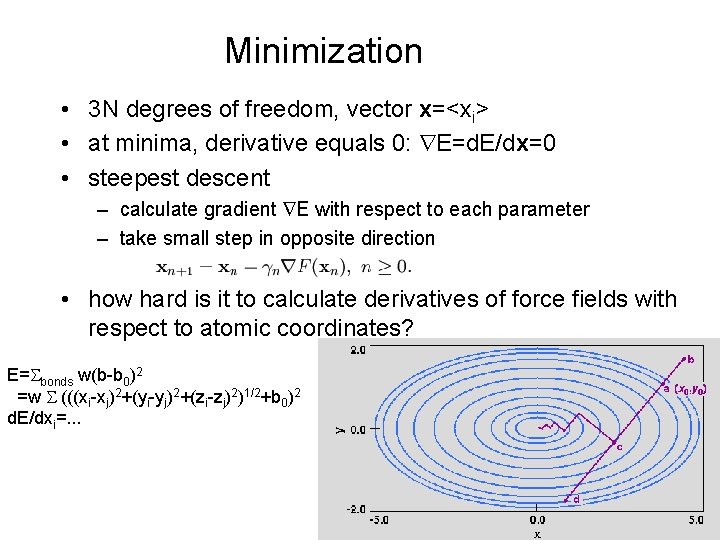
Minimization • 3 N degrees of freedom, vector x=<xi> • at minima, derivative equals 0: E=d. E/dx=0 • steepest descent – calculate gradient E with respect to each parameter – take small step in opposite direction • how hard is it to calculate derivatives of force fields with respect to atomic coordinates? E=Sbonds w(b-b 0)2 =w S (((xi-xj)2+(yi-yj)2+(zi-zj)2)1/2+b 0)2 d. E/dxi=. . .

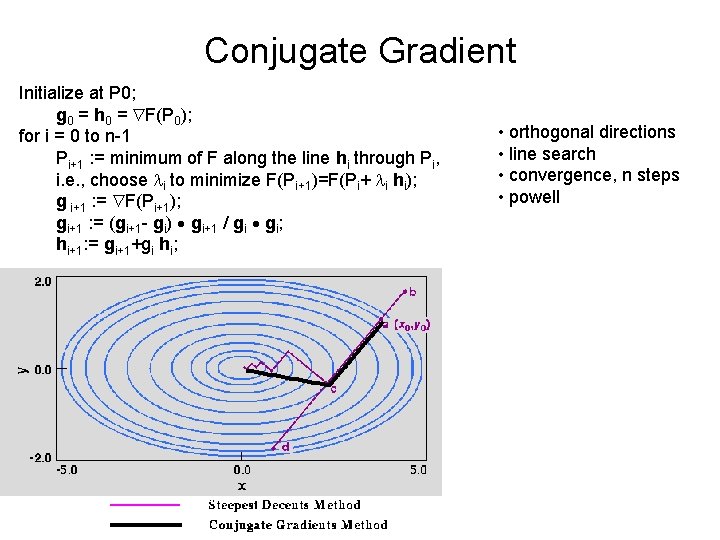
Conjugate Gradient Initialize at P 0; g 0 = h 0 = F(P 0); for i = 0 to n-1 Pi+1 : = minimum of F along the line hi through Pi, i. e. , choose li to minimize F(Pi+1)=F(Pi+ li hi); g i+1 : = F(Pi+1); gi+1 : = (gi+1 - gi) gi+1 / gi gi; hi+1: = gi+1+gi hi; • orthogonal directions • line search • convergence, n steps • powell

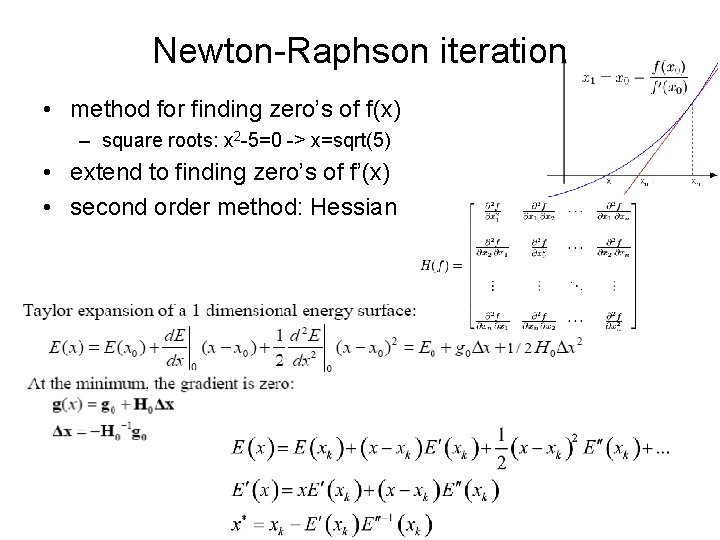
Newton-Raphson iteration • method for finding zero’s of f(x) – square roots: x 2 -5=0 -> x=sqrt(5) • extend to finding zero’s of f’(x) • second order method: Hessian

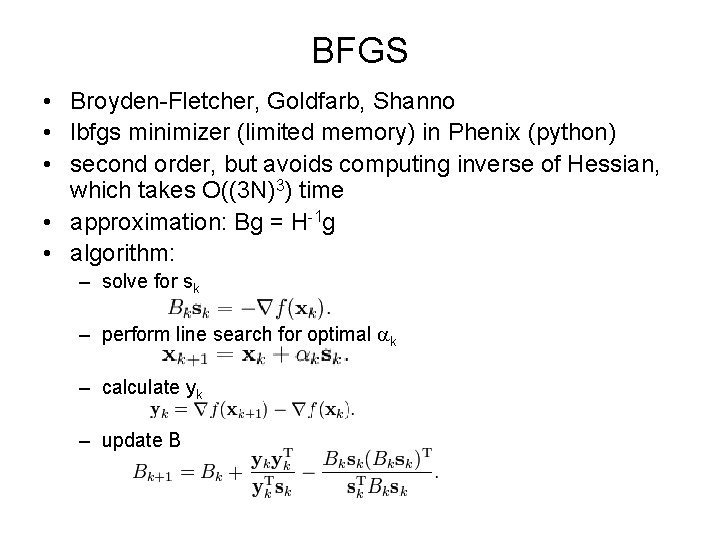
BFGS • Broyden-Fletcher, Goldfarb, Shanno • lbfgs minimizer (limited memory) in Phenix (python) • second order, but avoids computing inverse of Hessian, which takes O((3 N)3) time • approximation: Bg = H-1 g • algorithm: – solve for sk – perform line search for optimal ak – calculate yk – update B

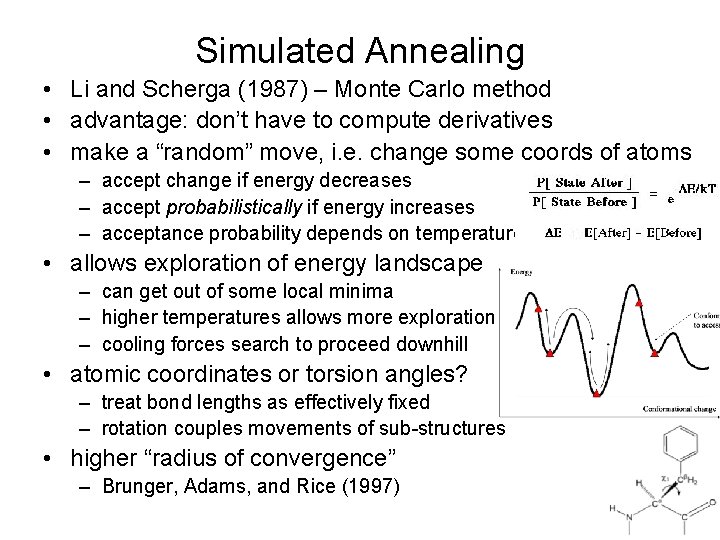
Simulated Annealing • Li and Scherga (1987) – Monte Carlo method • advantage: don’t have to compute derivatives • make a “random” move, i. e. change some coords of atoms – accept change if energy decreases – accept probabilistically if energy increases – acceptance probability depends on temperature • allows exploration of energy landscape – can get out of some local minima – higher temperatures allows more exploration – cooling forces search to proceed downhill • atomic coordinates or torsion angles? – treat bond lengths as effectively fixed – rotation couples movements of sub-structures • higher “radius of convergence” – Brunger, Adams, and Rice (1997)