Mitigating PreAnalytic Risk A Case Study Mary Jo





















- Slides: 39

Mitigating Pre-Analytic Risk – A Case Study Mary Jo Grodsky AACC July 2015

Objectives • How to use Quality Assurance to identify risk. • Identify mitigation options. • Documentation of risk mitigation. • Assess effectiveness of risk mitigation.

Who am I • Lab Coordinator, POCT for Orlando Regional Medical Center • Located in downtown Orlando, ORMC is Orlando Health’s flagship medical center, home to one of six teaching hospitals in the state and Central Florida’s only Level One Trauma center. ORMC specializes in trauma, cardiology, orthopedics, oncology, neurosciences, internal medicine and minimally invasive bariatric surgery.

Orlando Health Highlights: • • • One of Florida's most comprehensive private, not for profit healthcare networks Nine facilities: Five leading community hospitals, three nationally-recognized specialty hospitals and one world-class cancer center Number of beds: 1, 788 Central Florida's fifth largest employer Central Florida's only statutory teaching hospital offering graduate medical education A healthcare leader for nearly two million residents and thousands of international visitors annually People: • • • • Team members: 14, 310 Physicians: 2, 206 Medical Education Faculty: 88 Residents: 194 Residency programs: 8 Fellowship programs: 12 Patients Total admissions: (with newborns) 107, 405 Total births: 14, 705 Total surgeries: 62, 154 Total outpatient visits: 631, 764 Total emergency visits: 290, 275 Total trauma cases: 4, 786

Mitigating Pre-Analytic Risk Case Study-Bedside glucose pre-analytic risk • We have been mitigating pre-analytic risk for bedside glucose since I started in POCT approximately 15 years ago. • This is a summary of my journey of several years of mitigating the pre analytical risks associated with bedside glucose. • This case study will focus on specimen quality.

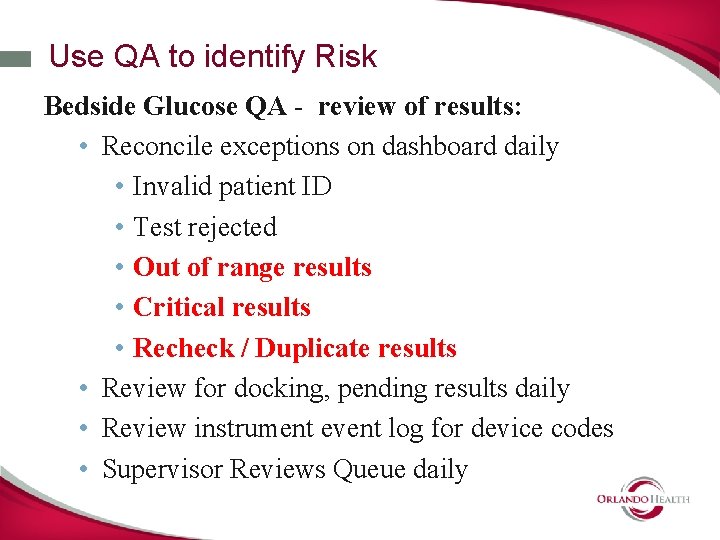
Use QA to identify Risk Bedside Glucose QA - review of results: • Reconcile exceptions on dashboard daily • Invalid patient ID • Test rejected • Out of range results • Critical results • Recheck / Duplicate results • Review for docking, pending results daily • Review instrument event log for device codes • Supervisor Reviews Queue daily

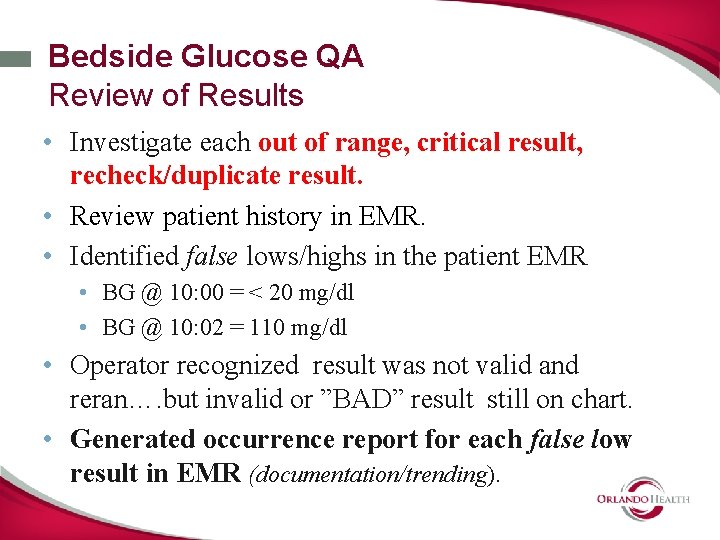
Bedside Glucose QA Review of Results • Investigate each out of range, critical result, recheck/duplicate result. • Review patient history in EMR. • Identified false lows/highs in the patient EMR • BG @ 10: 00 = < 20 mg/dl • BG @ 10: 02 = 110 mg/dl • Operator recognized result was not valid and reran…. but invalid or ”BAD” result still on chart. • Generated occurrence report for each false low result in EMR (documentation/trending).

Bedside Glucose QA Lab Occurrence Report

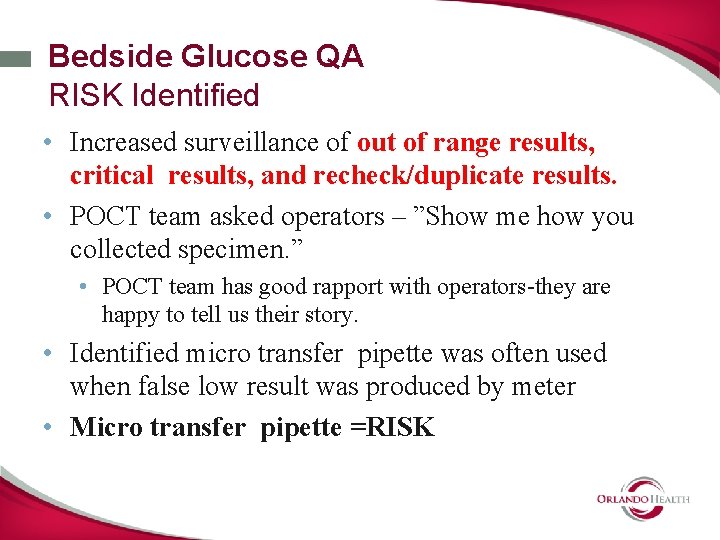
Bedside Glucose QA RISK Identified • Increased surveillance of out of range results, critical results, and recheck/duplicate results. • POCT team asked operators – ”Show me how you collected specimen. ” • POCT team has good rapport with operators-they are happy to tell us their story. • Identified micro transfer pipette was often used when false low result was produced by meter • Micro transfer pipette =RISK

POCT team rapport with operators

Bedside Glucose QA RISK Identified • Meeting with Nurse educators • POCT team has good rapport with Nurse educators and Nurse administrators • Share trending data for false results • Nurse educators agreed micro transfer pipettes were part of the problem but also educated POCC on vasopressor use and other peripheral perfusion issues with ICU patients. • Fingerstick = RISK

Bedside Glucose QA 2 Care Reviews Root Cause of issues identified as: • poor specimen choice, fingerstick in patient with peripheral circulation issues (blue finger tips) = false low result • Issues with line draw • Specimen diluted = false low result • Specimen contaminated= false high result • Line draw = RISK

Bedside Glucose QA 2 Care Reviews The Care review process brought bedside glucose specimen quality issues to the attention of the Quality Officer for the cardiology Service Line …. We now had a Physician Champion!

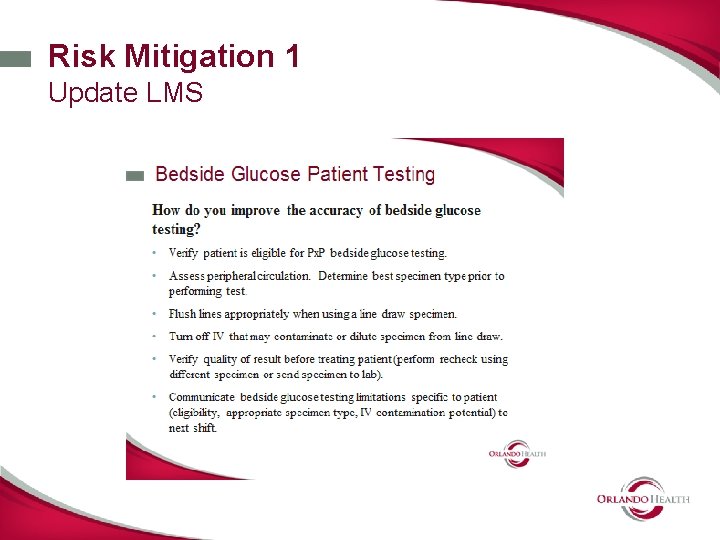
Bedside Glucose QA Care Review-what did we learn? • Fingersticks are not the appropriate specimen choice for patients with poor peripheral circulation. • Operators need education on line draw procedure • We need to enhance surveillance of erroneous results -trend all erroneous results in the POCT Data Management System- not just those in EMR.

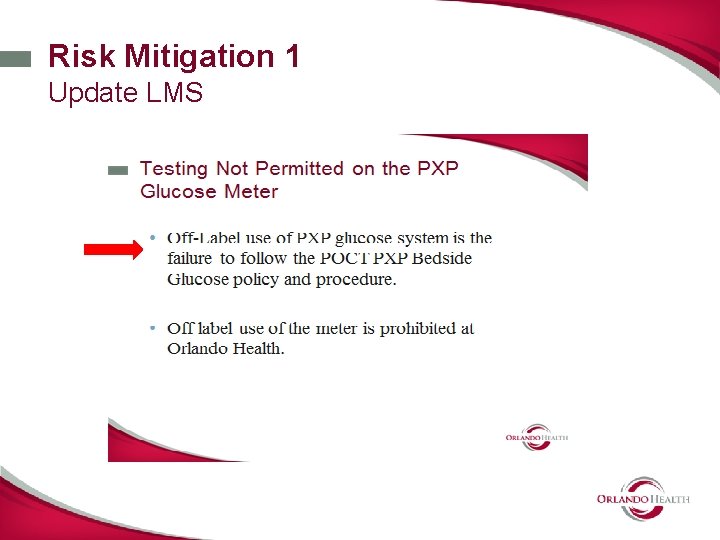
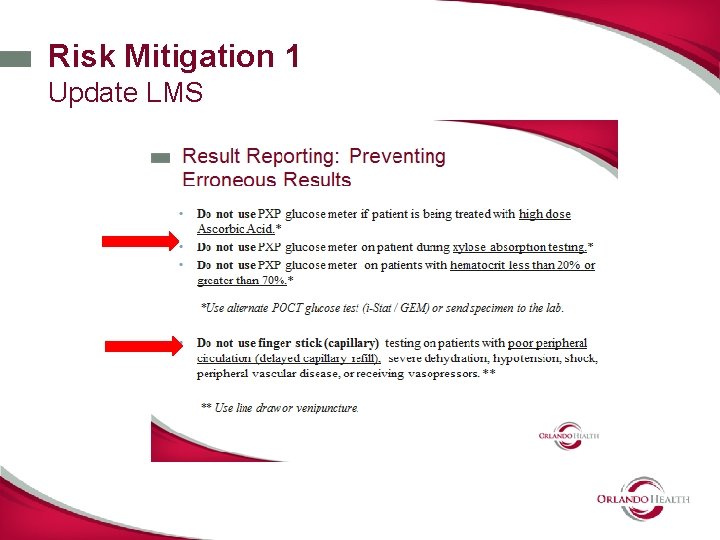
Identify Risk Mitigation Options 1. External Liquid QC will not mitigate the risks associated with specimen quality issues. 2. Needed other types of mitigations: • Removed micro transfer pipette from PAR carts • Enhanced new operator and annual training modules on Learning Management System. • Developed Educational Briefs and Safety Snip-it. • Added warning label to meter case. • Developed duplicate testing rule –electronic rule to prevent erroneous results on chart. • Added specimen quality to “operator lock out” guidelines • Activated Whole blood glucose on GEM 4000 in CVRR

Risk Mitigation 1 Update LMS

Risk Mitigation 1 Update LMS

Risk Mitigation 1 Update LMS

Risk Mitigation 2 Educational brief • Use LMS to distribute and track • Focus on choice of proper sample type and collection tips. • Reviews fingerstick vs line draw • Reviews avoiding contaminated or clotted specimen • Reviews placing meter in biohazard bag for isolation patient- eliminate need for micro transfer pipette • Reviews limitations of meter method (hct and ascorbic acid). • Highlights information critical to accurate results • Includes duplicate testing rule.

Risk Mitigation 2 Education Brief July 2014

Risk Mitigation 2 Education Brief May 2015

Risk Mitigation 3 Safety Snip-it

RISK Mitigation 4 Warning Label 2014 • Warning Label on each meter case

Risk Mitigation 4 Warning Label 2015

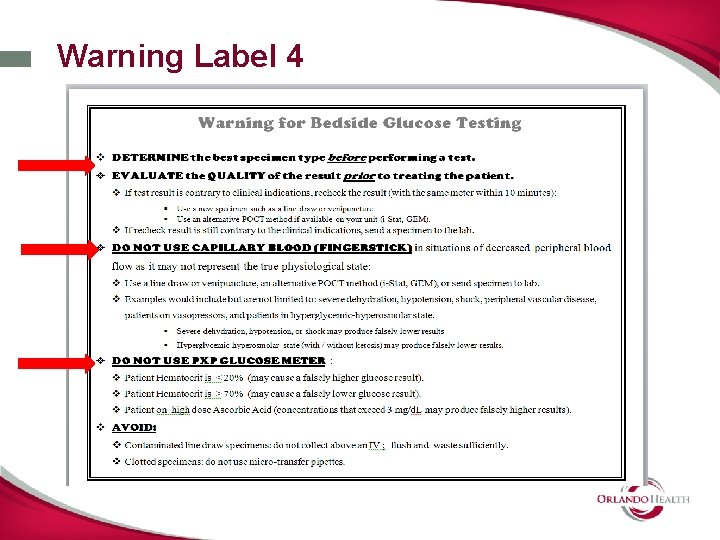
Warning Label 4

RISK Mitigation 5 Duplicate Testing Rule in the POCT DMS • Recheck Bedside Glucose • Second bedside glucose to verify the accuracy of the first result. • Duplicate Testing Rule will trigger in the DMS if: • • Recheck Bedside Glucose is performed on the same patient Recheck Bedside Glucose is performed on the same meter Recheck Bedside Glucose is performed within 10 minutes Meter is not docked until the Recheck Bedside Glucose test is completed; (meter should be docked after the Recheck Bedside Glucose is performed).

RISK Mitigation 5 Duplicate Testing Rule • When the Duplicate Testing Rule is triggered in the DMS: • The second result will post on the chart. • The first result will not post on the chart (the first result is considered the duplicate result). • The patient will be billed for one (1) bedside glucose test. The Duplicate Testing Rule ensures both an accurate medical record and accurate billing.

Risk Mitigation 6 Operator Lock-Out • Trend POCT errors for each operator (mislabeled, invalid ID, delay in docking, QC compliance, specimen quality) • 3 strikes and you lose access to POCT system • Bedside glucose • i-Stat • GEM 4000 • Must attend remedial training to regain access.

Documentation of Risk Mitigation • Include risk mitigations in QC Plan • (IQCP = RA+QC+QC) • P&P Updates • Stress proper sample type selection and collection technique • Use stronger language in the limitations of the meter in use ; replaced “avoid” with “Do Not”. • Off - Label use prohibited • Define critically ill as it relates to manufacture intended use and limitations with input from corporate P/P committee.

Documentation of Risk Mitigation • Track education compliance in the LMS • Document remedial training on training record /log • Monitor compliance • Audits • Occurrence trending • PI Score Card

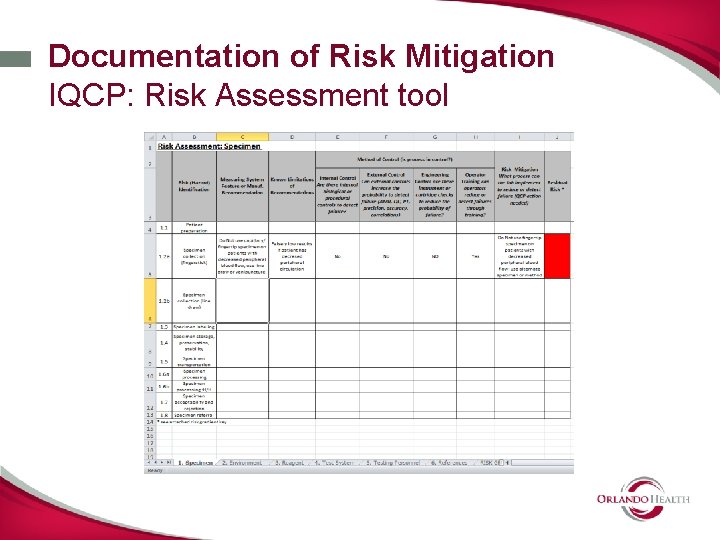
Documentation of Risk Mitigation IQCP: Risk Assessment tool

Assessment of Effectiveness Measurable improvement: • Micro transfer pipettes were gone. • We provided remedial training to 138 operators in 2014. • Duplicate testing rule kept “bad” results out of the EMR.

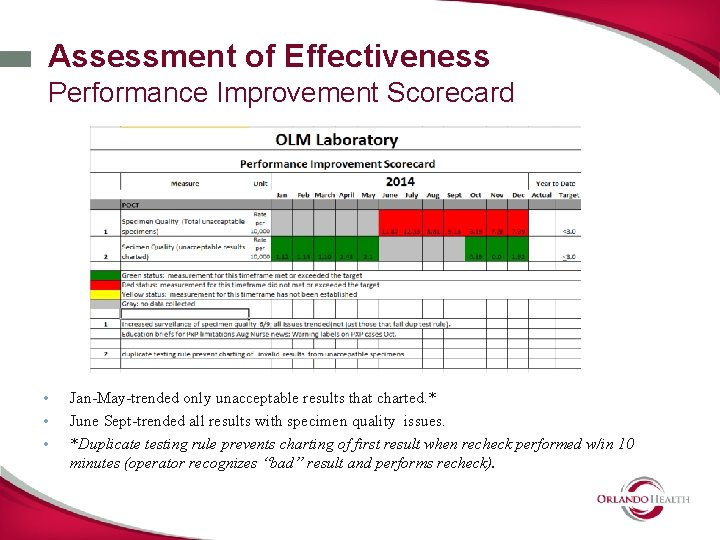
Assessment of Effectiveness Performance Improvement Scorecard • • • Jan-May-trended only unacceptable results that charted. * June Sept-trended all results with specimen quality issues. *Duplicate testing rule prevents charting of first result when recheck performed w/in 10 minutes (operator recognizes “bad” result and performs recheck).

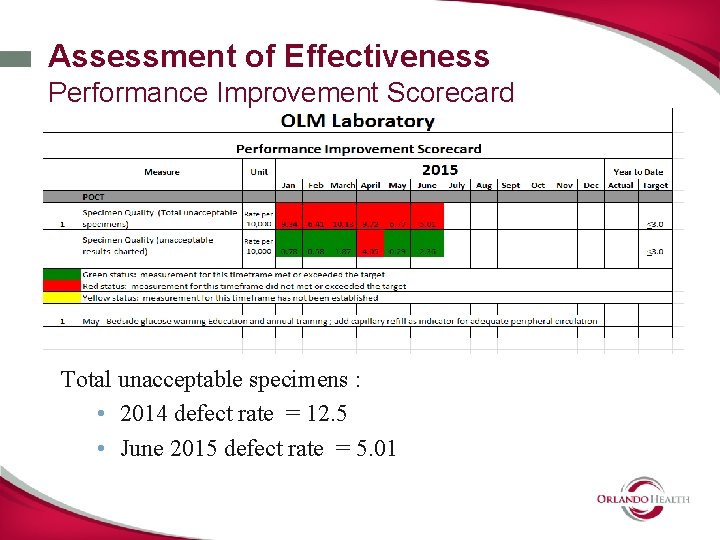
Assessment of Effectiveness Performance Improvement Scorecard Total unacceptable specimens : • 2014 defect rate = 12. 5 • June 2015 defect rate = 5. 01

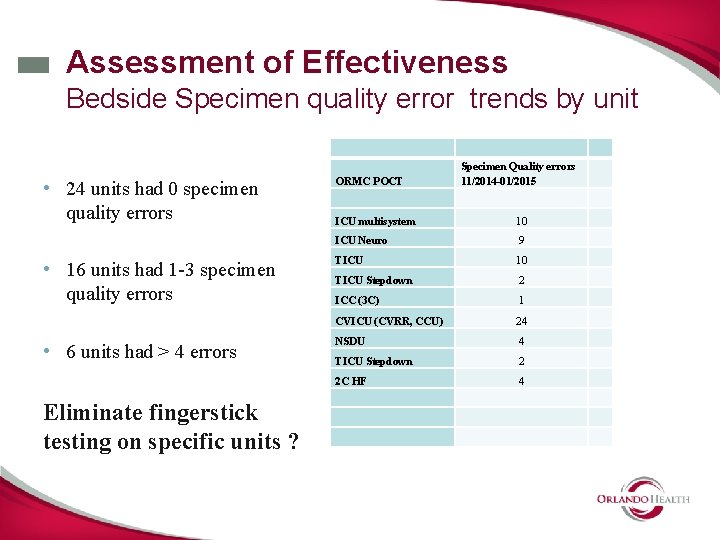
Assessment of Effectiveness Bedside Specimen quality error trends by unit • 24 units had 0 specimen quality errors • 16 units had 1 -3 specimen quality errors • 6 units had > 4 errors Eliminate fingerstick testing on specific units ? ORMC POCT Specimen Quality errors 11/2014 -01/2015 ICU multisystem 10 ICU Neuro 9 TICU 10 TICU Stepdown 2 ICC (3 C) 1 CVICU (CVRR, CCU) 24 NSDU 4 TICU Stepdown 2 2 C HF 4

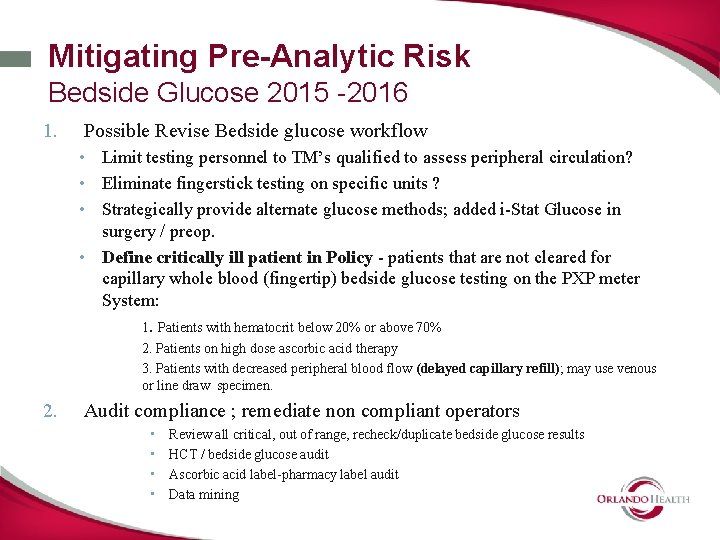
Mitigating Pre-Analytic Risk Bedside Glucose 2015 -2016 1. Possible Revise Bedside glucose workflow • Limit testing personnel to TM’s qualified to assess peripheral circulation? • Eliminate fingerstick testing on specific units ? • Strategically provide alternate glucose methods; added i-Stat Glucose in surgery / preop. • Define critically ill patient in Policy - patients that are not cleared for capillary whole blood (fingertip) bedside glucose testing on the PXP meter System: 1. Patients with hematocrit below 20% or above 70% 2. Patients on high dose ascorbic acid therapy 3. Patients with decreased peripheral blood flow (delayed capillary refill); may use venous or line draw specimen. 2. Audit compliance ; remediate non compliant operators • • Review all critical, out of range, recheck/duplicate bedside glucose results HCT / bedside glucose audit Ascorbic acid label-pharmacy label audit Data mining

Summary 1. Use QA Plan to identify risk • Review package insert - understand method limitations and specimen limitations • Review results; trend errors • Increase surveillance - talk to operators when trends appear 2. Identify mitigation options -include all players in mitigation • Education (education briefs, Safety Snip-it, annual review, warning label, remedial training) • Accountability (lock out) • Electronic tools (duplicate testing rule) • Alternate methods

Summary (cont. ) 3. Document risk mitigation • QC Plan • Policy / Procedure • LMS, education briefs 4. Assess effectiveness • Occurrence trending, audits • PI scorecard 5. Repeat steps 1 -4

Thank you Mary Jo Grodsky, BS, MLS (ASCP) Lab Coordinator Point of Care Testing Orlando Regional Medical Center, a part of Orlando Health 1414 Kuhl Ave. , MP 105 Orlando, FL 32806 maryjo. grodsky@orlandohealth. com tel: 321. 841. 6480 pager: 407. 980. 3790 fax: 407. 843. 5547