Metal ores and mining Ores are naturally occurring






- Slides: 12

Metal ores and mining • Ores are naturally occurring rocks found in the Earth’s crust. • They contain metal/metal compounds in sufficient amounts to make it worthwhile extracting them • Ores are mined and maybe concentrated before the metal is extracted and purified

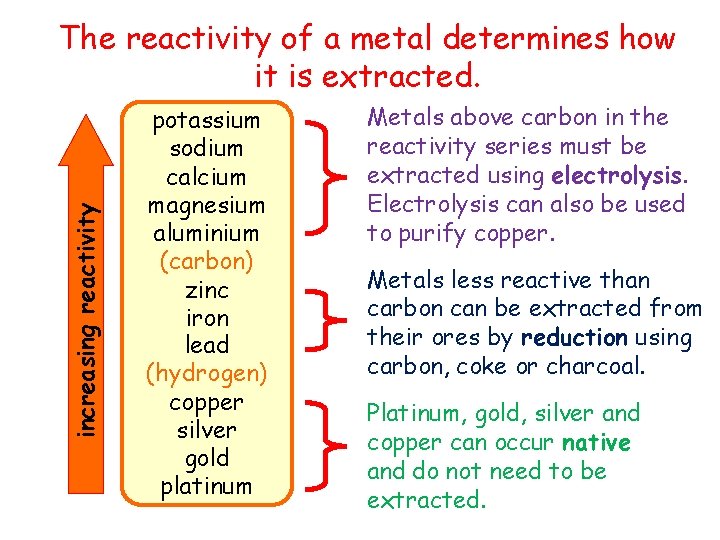
increasing reactivity The reactivity of a metal determines how it is extracted. potassium sodium calcium magnesium aluminium (carbon) zinc iron lead (hydrogen) copper silver gold platinum Metals above carbon in the reactivity series must be extracted using electrolysis. Electrolysis can also be used to purify copper. Metals less reactive than carbon can be extracted from their ores by reduction using carbon, coke or charcoal. Platinum, gold, silver and copper can occur native and do not need to be extracted.

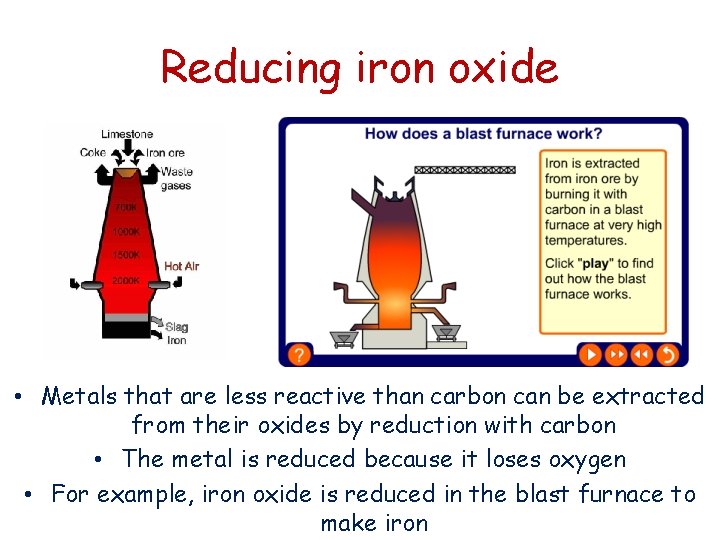
Reducing iron oxide • Metals that are less reactive than carbon can be extracted from their oxides by reduction with carbon • The metal is reduced because it loses oxygen • For example, iron oxide is reduced in the blast furnace to make iron

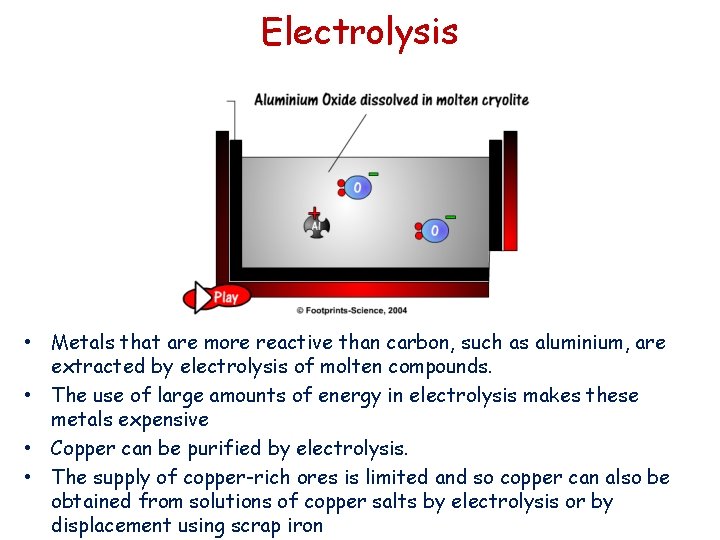
Electrolysis • Metals that are more reactive than carbon, such as aluminium, are extracted by electrolysis of molten compounds. • The use of large amounts of energy in electrolysis makes these metals expensive • Copper can be purified by electrolysis. • The supply of copper-rich ores is limited and so copper can also be obtained from solutions of copper salts by electrolysis or by displacement using scrap iron

Recycling metals • We should recycle metals because extracting them uses limited resources and is expensive in terms of energy and effects on the environment

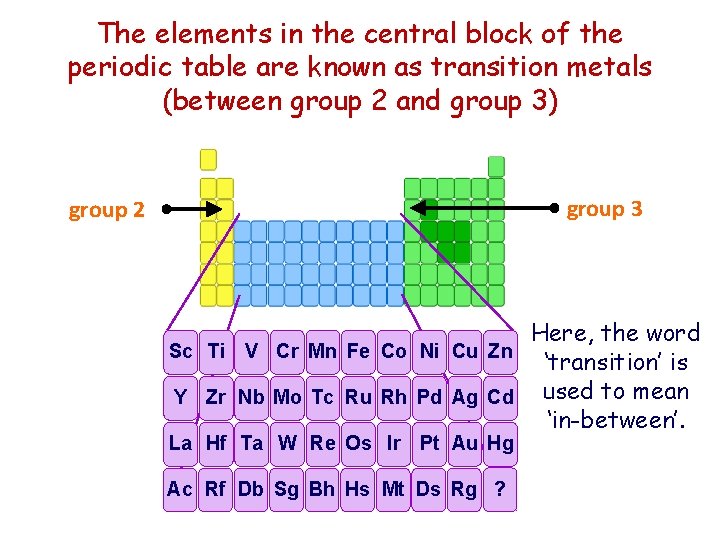
The elements in the central block of the periodic table are known as transition metals (between group 2 and group 3) group 3 group 2 Here, the word Sc Ti V Cr Mn Fe Co Ni Cu Zn ‘transition’ is Y Zr Nb Mo Tc Ru Rh Pd Ag Cd used to mean ‘in-between’. La Hf Ta W Re Os Ir Pt Au Hg Ac Rf Db Sg Bh Hs Mt Ds Rg ?

Properties of Transition metals Like other metals • They are good conductors of heat and electricity • They can be bent or hammered into shape • They are useful as structural materials and for making things that must allow heat or electricity to pass through them easily

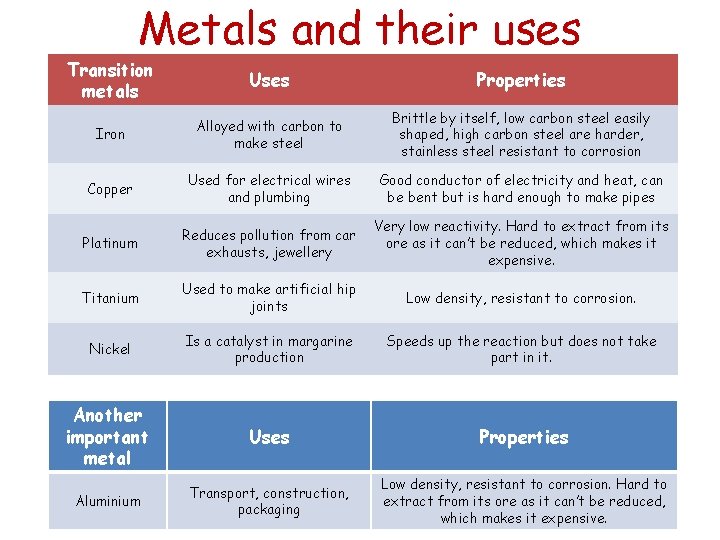
Metals and their uses Transition metals Uses Properties Iron Alloyed with carbon to make steel Brittle by itself, low carbon steel easily shaped, high carbon steel are harder, stainless steel resistant to corrosion Copper Used for electrical wires and plumbing Good conductor of electricity and heat, can be bent but is hard enough to make pipes Platinum Reduces pollution from car exhausts, jewellery Very low reactivity. Hard to extract from its ore as it can’t be reduced, which makes it expensive. Titanium Used to make artificial hip joints Low density, resistant to corrosion. Nickel Is a catalyst in margarine production Speeds up the reaction but does not take part in it. Another important metal Uses Properties Aluminium Transport, construction, packaging Low density, resistant to corrosion. Hard to extract from its ore as it can’t be reduced, which makes it expensive.

- Most metals in everyday use are alloys as pure copper, gold and aluminium are too soft for most uses - They are mixed with other similar metals to make them harder

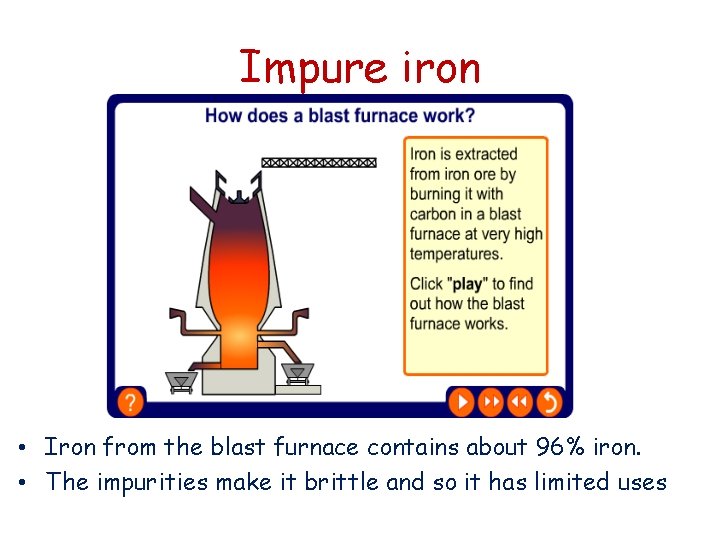
Impure iron • Iron from the blast furnace contains about 96% iron. • The impurities make it brittle and so it has limited uses

Alloys can be designed to have properties for specific uses Stainless steel Low carbon steel High carbon steel • Steels are alloys of iron and carbon. Some steels contain other metals. • Low carbon steels are easily shaped, high carbon steels are hard and stainless steels are resistant to corrosion

Some steels contain other metals.
 Insidan region jh
Insidan region jh Naturally occurring mineral
Naturally occurring mineral Heaviest naturally occurring element
Heaviest naturally occurring element Naturally occurring areas of hydrothermal resources
Naturally occurring areas of hydrothermal resources Naturally occurring areas of hydrothermal resources
Naturally occurring areas of hydrothermal resources Largest naturally occurring element
Largest naturally occurring element Cephalin structure
Cephalin structure Naturally occurring areas of hydrothermal resources
Naturally occurring areas of hydrothermal resources Naturally occurring antibodies
Naturally occurring antibodies Is a naturally occurring association among specific things
Is a naturally occurring association among specific things Naturally occurring inorganic solid material
Naturally occurring inorganic solid material Difference between strip mining and open pit mining
Difference between strip mining and open pit mining Web text mining
Web text mining






















