Medical device software apps Micael Johansson MEDQURE Therese











- Slides: 20

“Medical device software & apps” Micael Johansson MEDQURE & Therese Albinsson/Med. Qtech AB Copyright © 2017 , All rights reserved to MEDQURE IVS.

Introduction Micael Johansson, Senior Quality/Regulatory consultant and partner MEDQURE. 25 years in regulatory/quality/development. Background: - Test house/Notified Body (Intertek) - Product development of Critical care ventilator/anesthesia work station (MAQUET) - Senior consultancy (Various start-ups and international companies) Email : micael. johansson@nimio. se / mj@medqure. eu Tele: +46 -73 070 10 44 www. medqure. eu , MEDQURE IVS, Micael on Linked-in www. nimio. se www. medqure. eu 2

Introduction Med. Qtech AB is a consultant business within the medical device industry. We support and help our customers in the fields of quality and regulatory compliance. www. Med. Qtech. se • Therese Albinsson • 15 years within Medical Device Industry • Certification, ISO 13485, Compliance, MDD 93/42/EC, IVDD 98/79/EC • Facilitation of Risk Management, ISO 14971, Internal audits, supplier audits, Support in Medical Device CE-marketing process 3 www. medqure. eu 3

Po st-m Us Q s ual abi urv What is MEDQURE ark ity lity eill et sys an ce tem E , 13 C / R 485 D M : 20 • Course provider for medical device companies 16 • No memberships fee • Open and company specific courses C • Very experienced instructors leg hina isl I • Present in Denmark and Sweden, English courses…. D atio U • www. medqure. eu n RO HS s 2/R p y E t R 6 AC i Ap 0 m t l i i 6 s i a d k 0 & u H n b a 1 i a l s g t l e ri 149 em a a SW Interna c i p es in tion 71: ent l m C o a 2 , 0 u c l 1 a o v 2 i e B www. medqure. eu 4

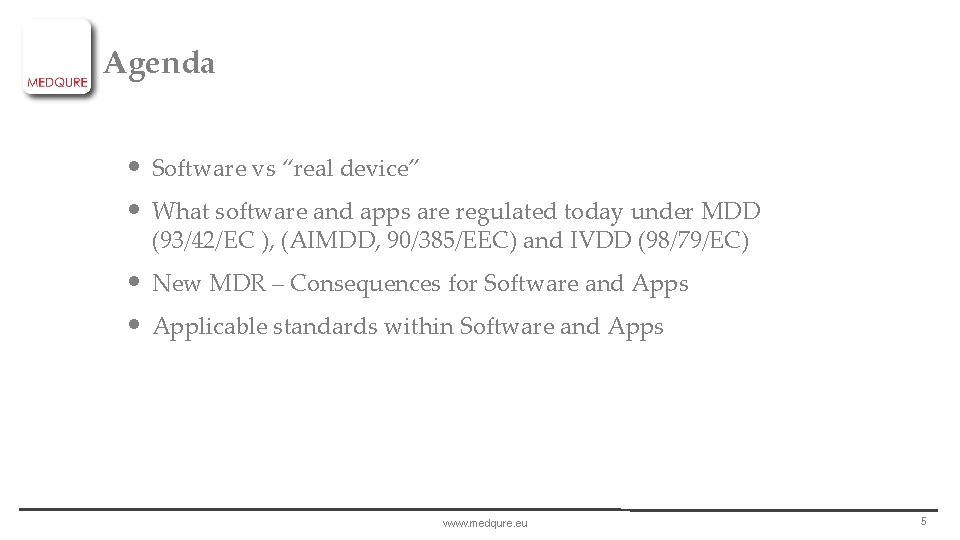
Agenda • Software vs “real device” • What software and apps are regulated today under MDD (93/42/EC ), (AIMDD, 90/385/EEC) and IVDD (98/79/EC) • New MDR – Consequences for Software and Apps • Applicable standards within Software and Apps www. medqure. eu 5

Mini-poll, MDR/IVDR • Have read the complete MDR/IVDR? • Have read a lot? • Have read some? • Have not read at all? 6 www. medqure. eu 6

Scope MDD, Software/App vs ”Real device” ? ? medical device’ means any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of: — diagnosis, prevention, monitoring, treatment or alleviation of disease, — diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap, — investigation, replacement or modification of the anatomy or of a physiological process, — control of conception, and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means www. medqure. eu 7

Software/App vs ”Real device” ? ? • No difference, “Intended use” essential • Embedded or stand alone? • Embedded together with device • • As for all devices, specific requirement/standards for SW Tricky with health/training apps/SW Active device, rule 9 -12 Often class I device in MDD acc to rule 12 www. medqure. eu 8

Software/App vs ”Real device” Examples of software that is medical device • Apps that handles data from the body: for example, MDD Temperature, pulse / oxygen combination, different types of ECG, (data input from e. g. a sensor or entered manually) • Blood Glucose Meters app IVD • Supports drug treatment by "coaching" the patient MDD www. medqure. eu 9

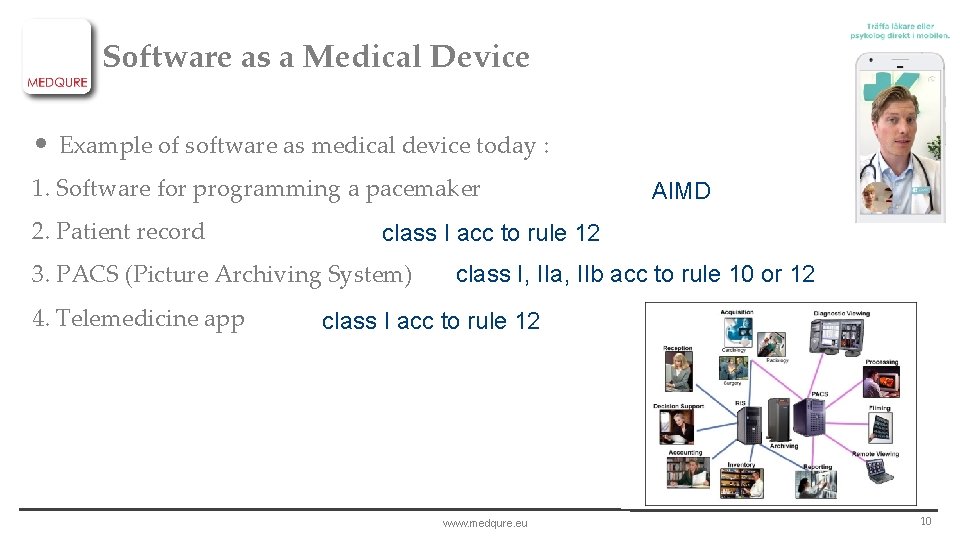
Software as a Medical Device • Example of software as medical device today : 1. Software for programming a pacemaker 2. Patient record class I acc to rule 12 3. PACS (Picture Archiving System) 4. Telemedicine app AIMD class I, IIa, IIb acc to rule 10 or 12 class I acc to rule 12 www. medqure. eu 10

New regulations for Medical devices 26 th May 2017 MDR 2017/745 3 year IVDR 2017/746 5 year www. medqure. eu 11

MDR vs MDD, SW, overview - Search software - MDD, approx 5 matches - MDR, 54 matches - Some news in MDR - New rule for classification, rule 11 - In general enhanced requirements on software - Mobile platforms - IT-security - Proof of verification/validation www. medqure. eu 12

Classification, MDR, SW NEW Rule 11 Software intended to provide information which is used to take decisions with diagnosis or therapeutic purposes is classified as class IIa, except if such decisions have an impact that may cause: — death or an irreversible deterioration of a person's state of health, in which case it is in class III; or — a serious deterioration of a person's state of health or a surgical intervention, in which case it is classified as class IIb. Software intended to monitor physiological processes is classified as class IIa, except if it is intended for monitoring of vital physiological parameters, where the nature of variations of those parameters is such that it could result in immediate danger to the patient, in which case it is classified as class IIb. All other software is classified as class I. www. medqure. eu 15

COCIR, rule 11 COCIR is the European Trade Association representing the medical imaging, radiotherapy, health ICT and electromedical industries. ……… COCIR is unique as it brings together the healthcare, IT and telecommunications industries…… Source: COCIR www. medqure. eu 16

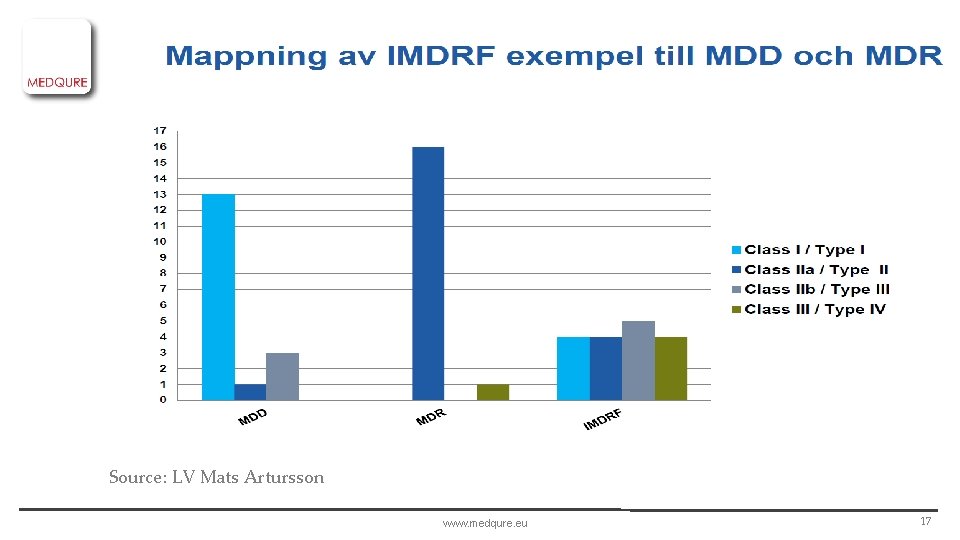
Source: LV Mats Artursson www. medqure. eu 17

Requirements on SW, annex I, MDR/IVDR 17. Electronic programmable systems — devices that incorporate electronic programmable systems and software that are devices in themselves 17. 1. Devices that incorporate electronic programmable systems, including software, or software that are devices in themselves, shall be designed to ensure repeatability, reliability and performance in line with their intended use In the event of a single fault condition, appropriate means shall be adopted to eliminate or reduce as far as possible consequent risks or impairment of performance. 17. 2. For devices that incorporate software or for software that are devices in themselves, the software shall be developed and manufactured in accordance with the state of the art taking into account the principles of development life cycle, risk management, including information security, verification and validation GDPR EN 62304 + A 1 www. medqure. eu 19

Requirements on SW, annex I 17. 3. Software referred to in this Section that is intended to be used in combination with mobile computing platforms shall be designed and manufactured taking into account the specific features of the mobile platform (e. g. size and contrast ratio of the screen) and the external factors related to their use (varying environment as regards level of light or noise). noise 17. 4. Manufacturers shall set out minimum requirements concerning hardware, IT networks characteristics and IT security measures, including protection against unauthorised access, necessary to run the software as intended. www. medqure. eu 20

In technical file, § 6 Annex II, MDR/IVDR — software verification and validation (describing the software design and development process and evidence of the validation of the software, as used in the finished device. This information shall typically include the summary results of all verification, validation and testing performed both in-house and in a simulated or actual user environment prior to final release It shall also address all of the different hardware configurations and, where applicable, operating systems identified in the information supplied by the manufacturer) www. medqure. eu 22

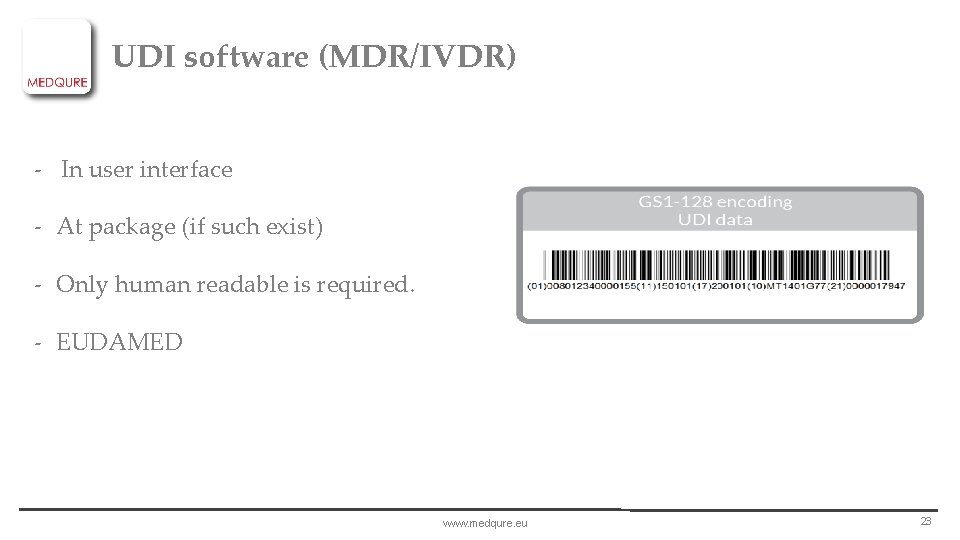
UDI software (MDR/IVDR) - In user interface - At package (if such exist) - Only human readable is required. - EUDAMED www. medqure. eu 23

Questions? Introduction EN 60601 -series overview - Including all collaterals - Products standards - IEC 60601 international Copyright © 2017 , All rights reserved to MEDQURE IVS. . www. medqure. eu 24
 Consumer apps vs enterprise apps
Consumer apps vs enterprise apps Hardware output
Hardware output Medical devices software fmea
Medical devices software fmea Oefeningen sinding larsen johansson
Oefeningen sinding larsen johansson Christer johansson stockholm
Christer johansson stockholm Scarlett johansson anniversaire
Scarlett johansson anniversaire Dr olga johansson
Dr olga johansson Cheerios scarlett johansson
Cheerios scarlett johansson Frans johansson medici effect
Frans johansson medici effect Toril johansson
Toril johansson Nina monsef johansson
Nina monsef johansson Natalia la porta wiki
Natalia la porta wiki Freedom photonics
Freedom photonics Frans johansson medici effect
Frans johansson medici effect Therese camilleri
Therese camilleri Military brotherhood tattoos
Military brotherhood tattoos St. therese of lisieux facts
St. therese of lisieux facts Therese meers
Therese meers Schema christinaskolan
Schema christinaskolan Rauquin
Rauquin Therese hughes
Therese hughes