MATHEMATICS THE LANGUAGE OF SCIENCE SIGNIFICANT FIGURES Defined

















- Slides: 37

MATHEMATICS THE LANGUAGE OF SCIENCE

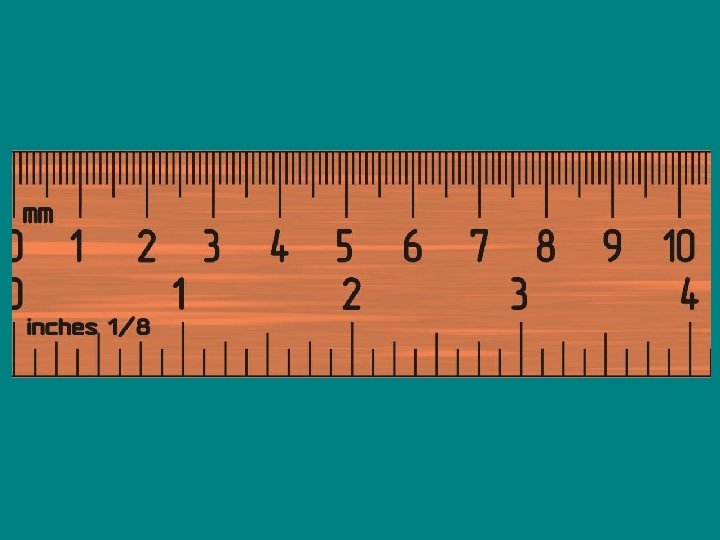
SIGNIFICANT FIGURES • Defined as all of the digits that can be read directly from the instrument used in making the measurement plus one uncertain digit that is obtained by estimating the fraction of the smallest division of the instrument’s scale


Significant figures • Rules: – NON-ZERO digits • 1, 2, 3, 4, 5, 6, 7. & 9 are always significant. – DIGIT ZERO • Zero may or may not be significant, depending on whether they mark the decimal point or indicate a measured value.

Significant figures • The DIGIT zeros – Leading zeros: • Zeros located at the beginning of a number are NEVER significant. They merely locate the decimal point. – Ex. 0. 0254 – 3 significant numbers (2, 5, 4) – Confines zeros • Zeros located between non-zero digits are ALWAYS significant. – Ex. 104. 6 m – 4 significant numbers (1, 0, 4, 6)

Significant figures • The digit zero – Trailing zeros: • Zeros located at the end of a number are significant only if the number has an explicitly shown decimal. – Ex. 2705. 00 – 6 significant numbers ( 2, 7, 0, 5, 0, 0) • In whole numbers without a decimal point that end in one or more zeros, the zeros may not be significant – Ex. 5000 – 1 significant number (5)

Significant figures • The digit zeros – Trailing zeros • Numbers expressed in scientific notation with zero, follows the rule in decimal number. – Ex. 5. 0 X 105 – 2 significant numbers (5, 0) – 2. 30 X 10 -8 – 3 significant numbers (2, 3, 0)

HOW MANY SIGNIFICANT DIGITS ARE THERE? 1. 25 2. 200. 5 3. 0. 0025 4. 0. 0250 5. 300 6. 1. 48 7. 800 x 10 -4 8. 0. 4904 9. 980476 10. 6739. 30 x 10 -5

Rounding off numbers 1. If the next digit after the last significant figure is 5 or greater, round up: Increase the last significant figure by 1. ex. 2. 136 become 2. 14 rounded to 3 significant figures

Rounding off numbers 2. If the next digit after the last significant figure is less than 5, round down: do not change the last significant figure. ex. 2. 132 become 2. 13 rounded to 3 significant figures

Round off the following numbers to the nearest 10 th 1. 2469. 4508 2. 1. 805 3. 4. 3849 4. 487. 554 5. 89320. 444 6. 13. 873 7. 3245. 8739 8. 45. 135 9. 499. 502 10. 4. 0009

ACCURACY & PRECISION • Reasons why the measurement or physical quantity is always subject to some degrees of uncertainty: 1. The limitations inherent in the construction of the measuring instrument. 2. The conditions under which the measurement is made. 3. The different ways in which the person uses or read the measuring instrument.

ACCURACY • Refers to the closeness of a measurement to the accepted value for a specific physical quantity. It is expressed as either an absolute error or a relative error.

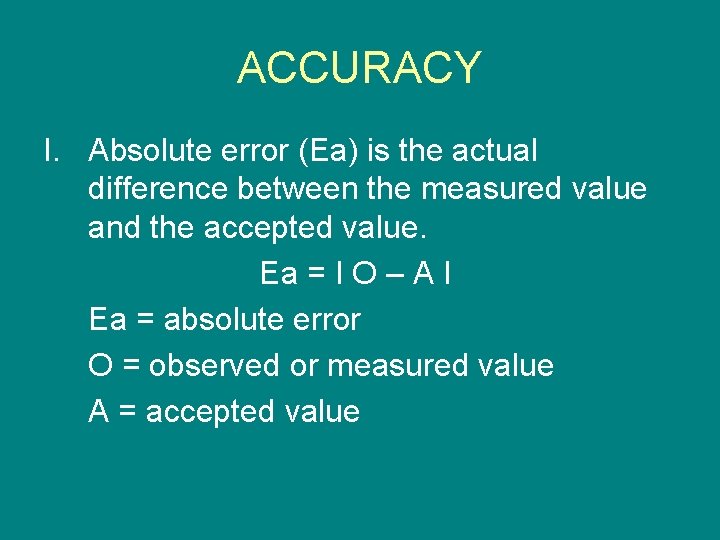
ACCURACY I. Absolute error (Ea) is the actual difference between the measured value and the accepted value. Ea = I O – A I Ea = absolute error O = observed or measured value A = accepted value

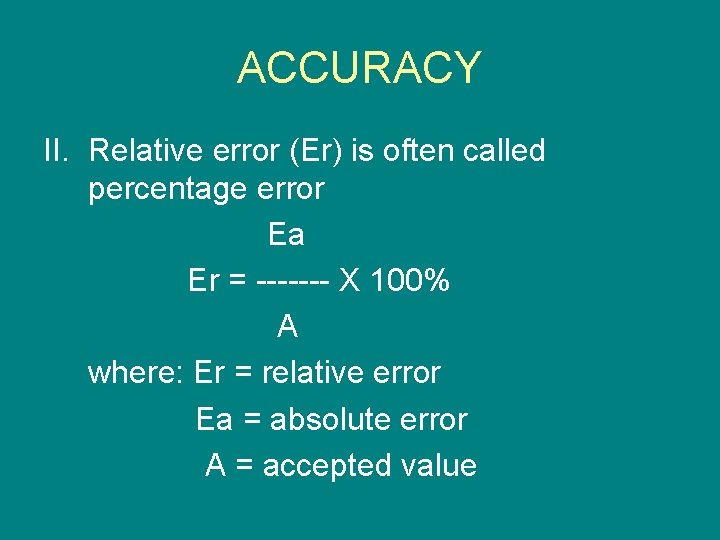
ACCURACY II. Relative error (Er) is often called percentage error Ea Er = ------- X 100% A where: Er = relative error Ea = absolute error A = accepted value

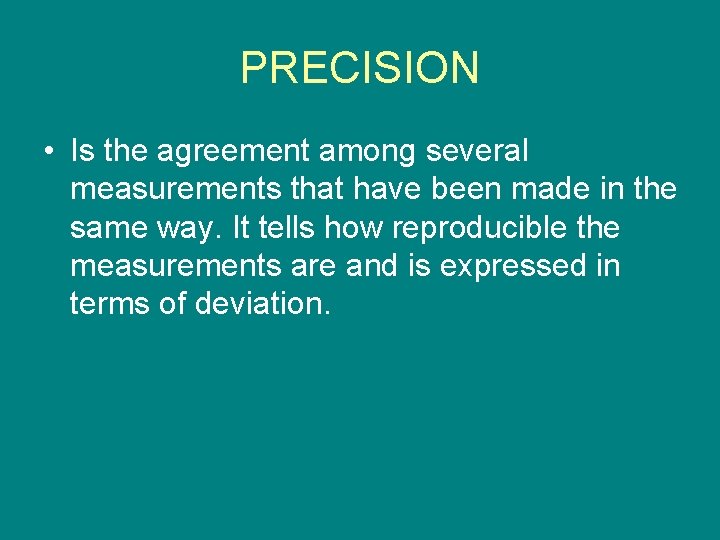
PRECISION • Is the agreement among several measurements that have been made in the same way. It tells how reproducible the measurements are and is expressed in terms of deviation.

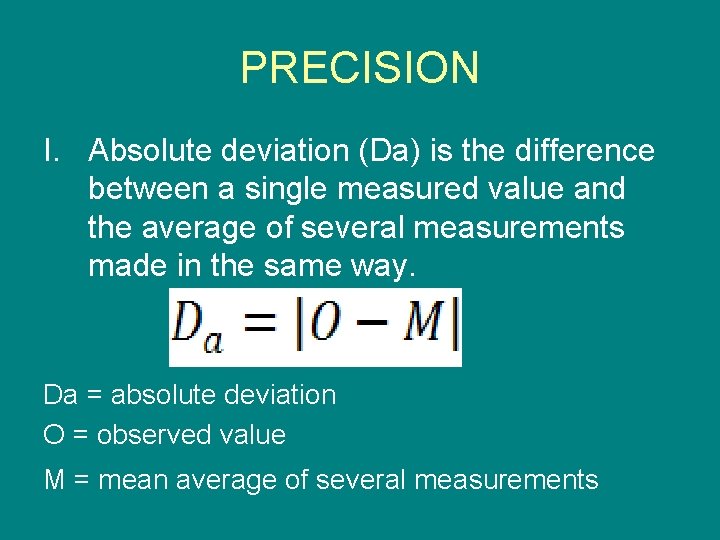
PRECISION I. Absolute deviation (Da) is the difference between a single measured value and the average of several measurements made in the same way. Da = absolute deviation O = observed value M = mean average of several measurements

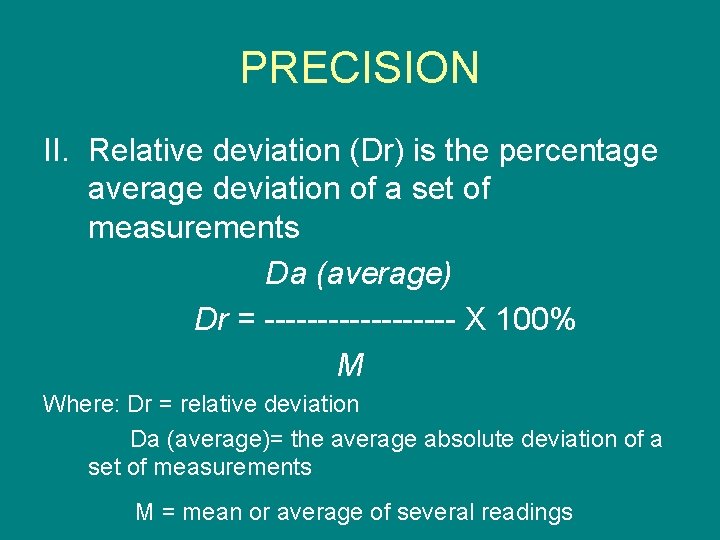
PRECISION II. Relative deviation (Dr) is the percentage average deviation of a set of measurements Da (average) Dr = --------- X 100% M Where: Dr = relative deviation Da (average)= the average absolute deviation of a set of measurements M = mean or average of several readings

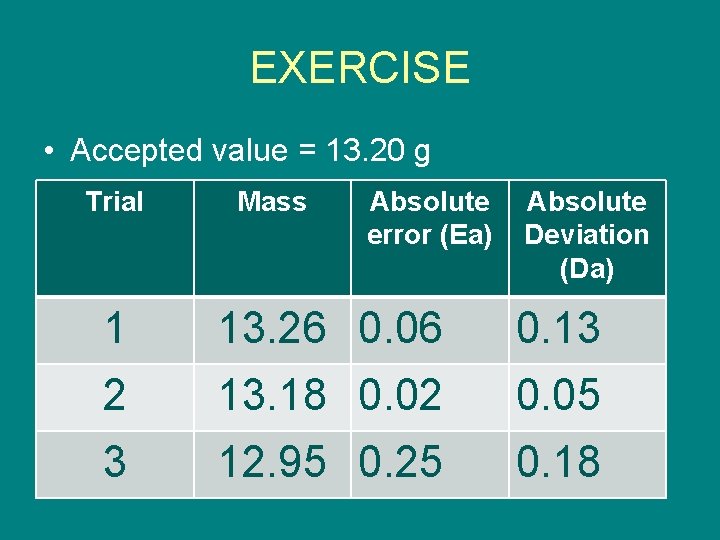
EXERCISE • Accepted value = 13. 20 g Trial Mass Absolute error (Ea) Absolute Deviation (Da) 1 13. 26 0. 06 0. 13 2 13. 18 0. 02 0. 05 3 12. 95 0. 25 0. 18

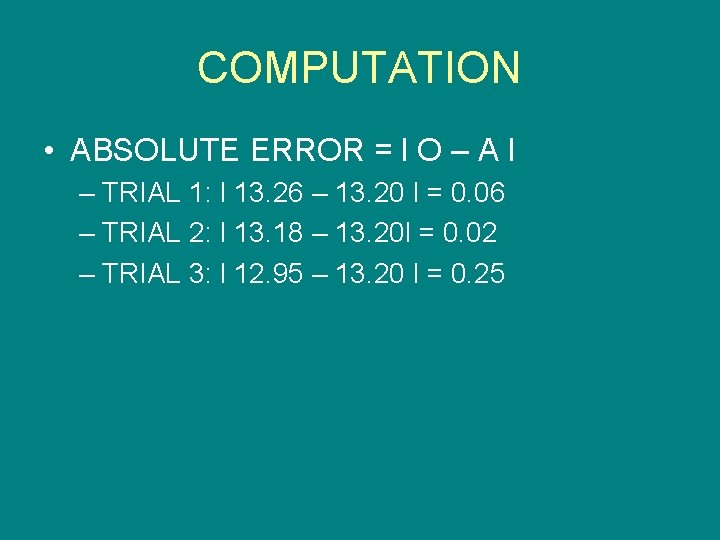
COMPUTATION • ABSOLUTE ERROR = l O – A l – TRIAL 1: l 13. 26 – 13. 20 l = 0. 06 – TRIAL 2: l 13. 18 – 13. 20 l = 0. 02 – TRIAL 3: l 12. 95 – 13. 20 l = 0. 25

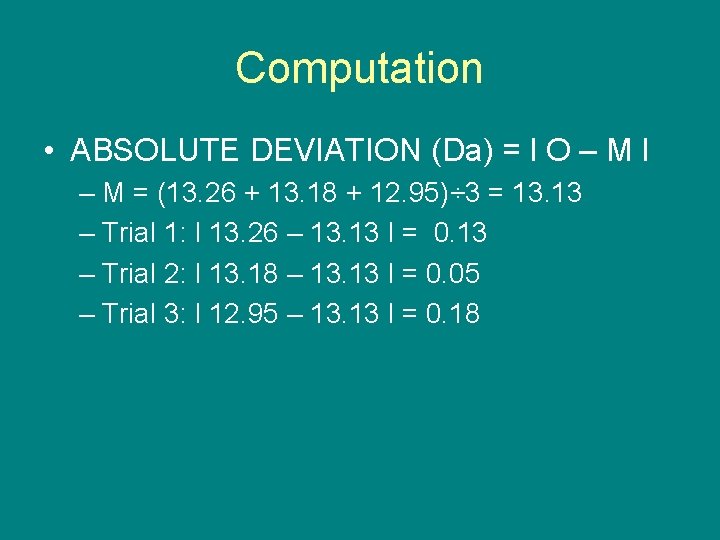
Computation • ABSOLUTE DEVIATION (Da) = l O – M l – M = (13. 26 + 13. 18 + 12. 95)÷ 3 = 13. 13 – Trial 1: l 13. 26 – 13. 13 l = 0. 13 – Trial 2: l 13. 18 – 13. 13 l = 0. 05 – Trial 3: l 12. 95 – 13. 13 l = 0. 18

EXERCISE Based on the given on slide number 19, COMPUTE THE FOLLOWING: 1. Er of trial 1 and 3 Er of trial 1 = (0. 06 ÷ 13. 20) X 100% = 0. 45% Er of trial 3 = (0. 25 ÷ 13. 20) X 100% = 1. 89% 1. Average of Mass: Answer = 13. 13 2. Dr of the data: [Da (average) ÷ M] X 100% {[( 0. 13 + 0. 05 + 0. 18) ÷ 3] ÷ 13. 13} X 100% = 0. 91%

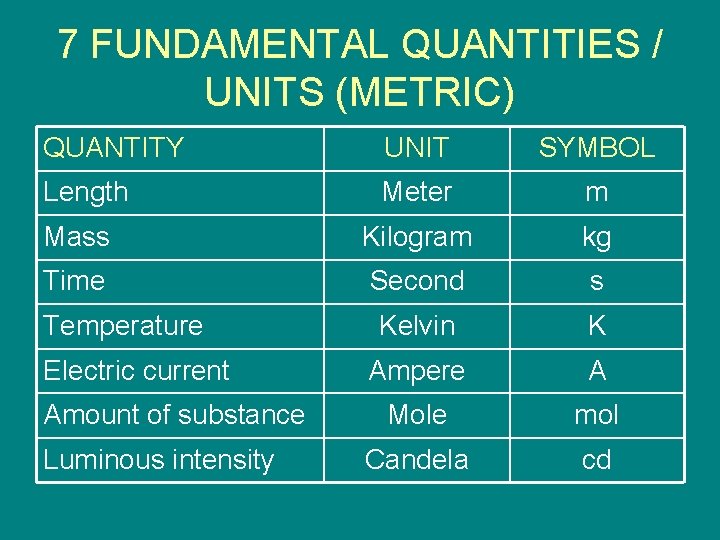
FUNDAMENTAL AND DERIVED QUANTITIES/UNITS 1. FUNDAMENTAL QUANTITIES / UNITS – The simplest quantities and units that are convenient to use as the basis for explaining or defining other quantities and units – 7 fundamental quantities and units (metric)

7 FUNDAMENTAL QUANTITIES / UNITS (METRIC) QUANTITY UNIT SYMBOL Length Meter m Mass Kilogram kg Time Second s Kelvin K Ampere A Mole mol Candela cd Temperature Electric current Amount of substance Luminous intensity

Derived quantities / units • Are quantities and units defined in terms of the fundamental quantities and units are said to be derived quantities.

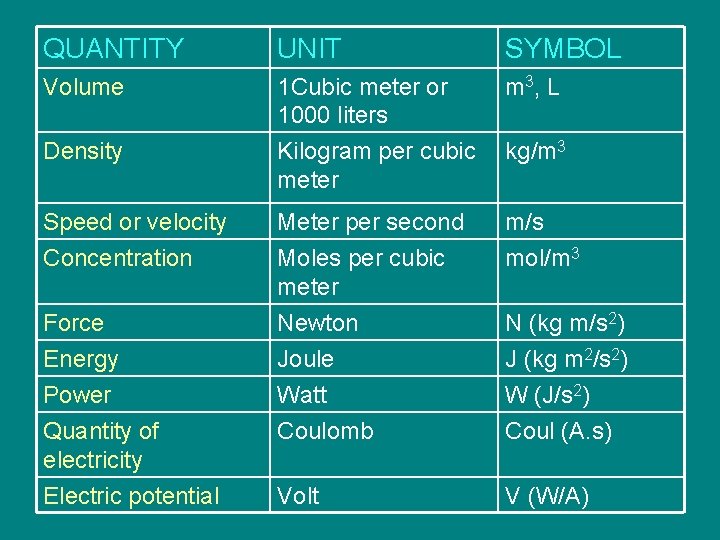
QUANTITY UNIT SYMBOL Volume 1 Cubic meter or 1000 liters Kilogram per cubic meter m 3 , L Meter per second Moles per cubic meter Newton Joule Watt Coulomb m/s mol/m 3 Volt V (W/A) Density Speed or velocity Concentration Force Energy Power Quantity of electricity Electric potential kg/m 3 N (kg m/s 2) J (kg m 2/s 2) W (J/s 2) Coul (A. s)

SYSTEM OF UNITS • current: International System of Units (SI) – To standardize and simplify measurements and promote advances in science and technology • Metric system – 2 system of untis: – MKS (meter, kilogram & second) – CGS (centimeter, gram & second)

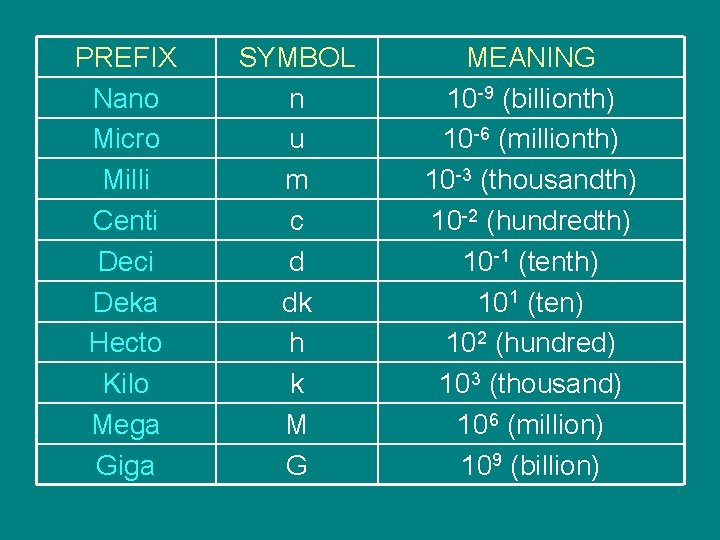
PREFIX Nano Micro Milli Centi Deci Deka Hecto Kilo Mega Giga SYMBOL n u m c d dk h k M G MEANING 10 -9 (billionth) 10 -6 (millionth) 10 -3 (thousandth) 10 -2 (hundredth) 10 -1 (tenth) 101 (ten) 102 (hundred) 103 (thousand) 106 (million) 109 (billion)

CONVERSION OF UNITS 1. Conversion factors • Characteristics of conversion factors: • Ratios that specify how units are related to each other • Derived from equations that relate units 1 minute = 60 seconds • Come in pairs, one member of one pair being the reciprocal of the other 1 min/60 sec & 60 sec/1 min Given quantity X conversion factor = desired quantity

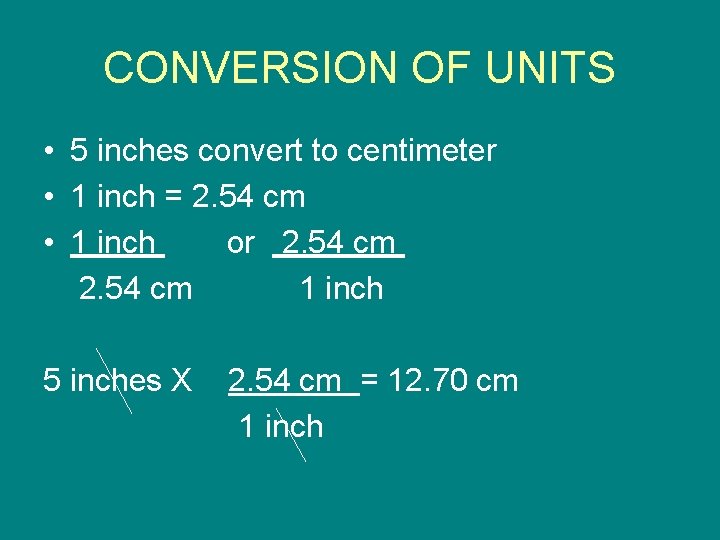
CONVERSION OF UNITS • 5 inches convert to centimeter • 1 inch = 2. 54 cm • 1 inch or 2. 54 cm 1 inch 5 inches X 2. 54 cm = 12. 70 cm 1 inch

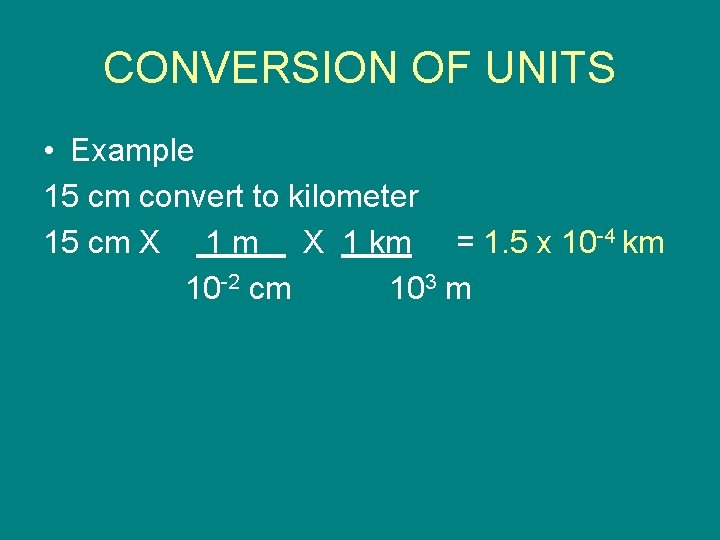
CONVERSION OF UNITS • Example 15 cm convert to kilometer 15 cm X 1 km = 1. 5 x 10 -4 km 10 -2 cm 103 m

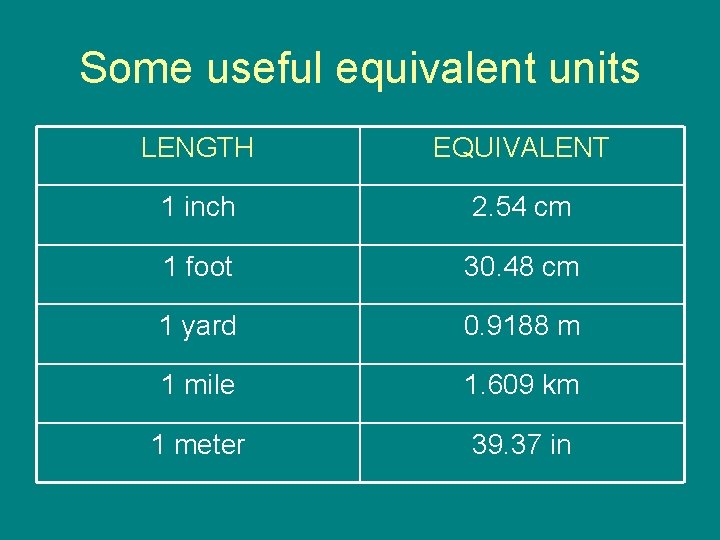
Some useful equivalent units LENGTH EQUIVALENT 1 inch 2. 54 cm 1 foot 30. 48 cm 1 yard 0. 9188 m 1 mile 1. 609 km 1 meter 39. 37 in

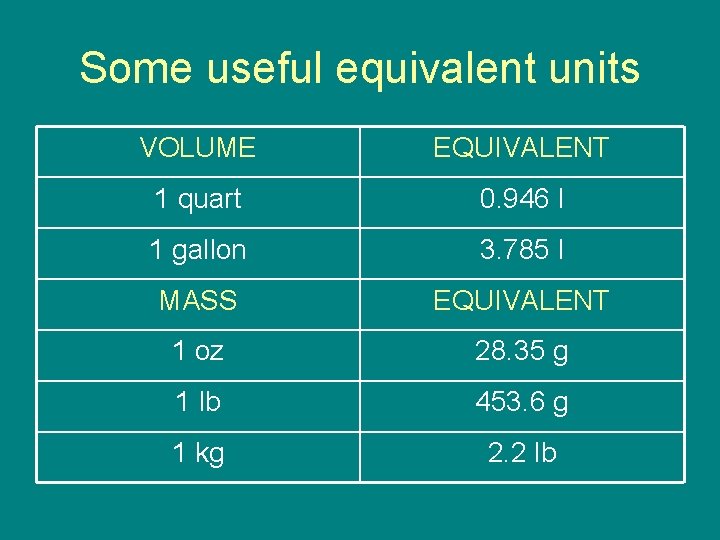
Some useful equivalent units VOLUME EQUIVALENT 1 quart 0. 946 l 1 gallon 3. 785 l MASS EQUIVALENT 1 oz 28. 35 g 1 lb 453. 6 g 1 kg 2. 2 lb

EXERCISE a. Convert 1. 25 km to cm = 125, 000 b. How many liters are there in exactly 25 m 3 ANS: 25000 LITERS (conversion factor is 1000 liters = 1 cubic meter) c. Convert 2 yards to mm d. Express 5 ft and 3 inch in cm e. Convert 130 lbs to kg

CONVERSION OF UNITS 2. FOR TEMPERATURE • The SI unit of temperature is the Kelvin (K). However, thermometer is never marked with the kelvin scale. • To convert Celsius to Fahrenheit Tf = 1. 8 Tc + 32°F

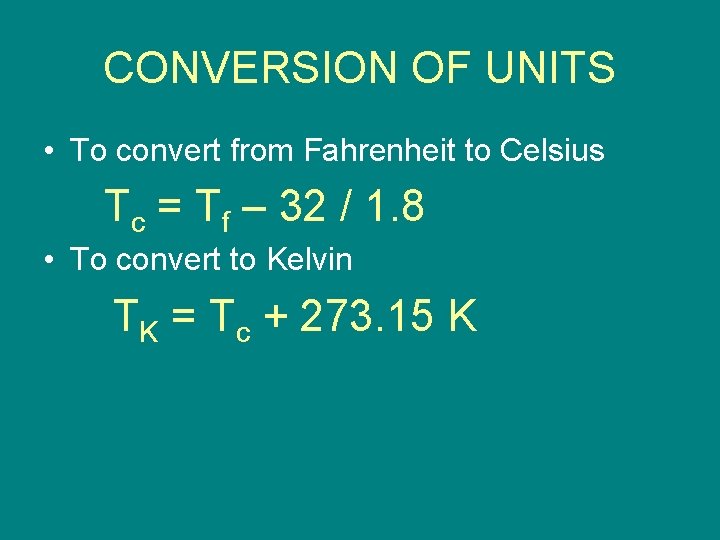
CONVERSION OF UNITS • To convert from Fahrenheit to Celsius Tc = Tf – 32 / 1. 8 • To convert to Kelvin TK = Tc + 273. 15 K

EXERCISE a. Normal body temperature is 37° C, convert it to °F b. Nitrogen boils at -196 °C. What is this temperature in Kelvin scale?