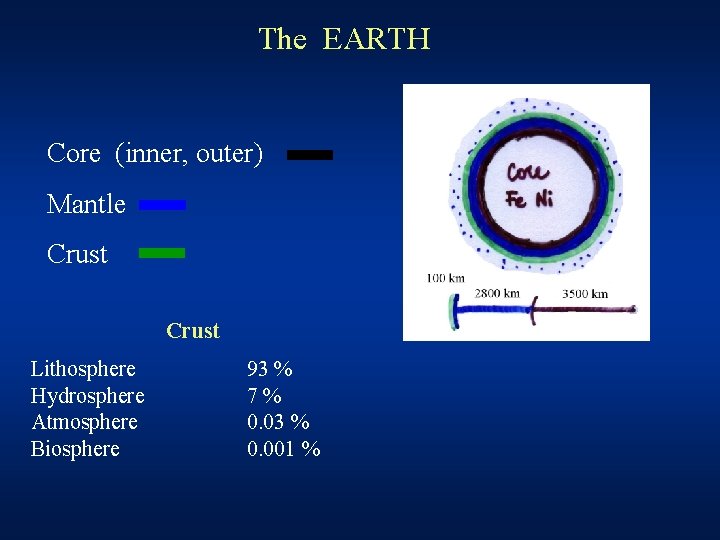
Life Chemistry The EARTH Core inner outer Mantle









- Slides: 28

Life Chemistry

The EARTH Core (inner, outer) Mantle Crust Lithosphere Hydrosphere Atmosphere Biosphere 93 % 7% 0. 03 % 0. 001 %

The elementary composition (%) Earth crust Human body O 49. 5 62. 4 Si 25. 7 - Al 7. 5 - Fe 4. 7 0. 005 H 0. 9 9. 8 P 0. 12 1. 0 C 0. 09 21. 1 S 0. 06 0. 16 N 0. 03 3. 1 - elementary composition of the human body is very different from that of lithosphere

Specific selection of some elements for the biological uses % in human body % in the Earth crust C 243 N 103 H 11 P 8 S 2. 7 O 1. 26 Fe 0. 001 Al 0. 000 13 Si 0. 000 04 - some chemical elements (C, H, N, O, . . . ) are "better" than others to make up molecules of living organisms

- most abundant elements of the Earth core: Fe, Ni - most abundant elements of the Earth crust: O, Si, Al, Fe - most abundant elements in living organisms: O, C, H, N - oxygen is the most abundant element both on the Earth surface and in human body

The atmosphere of the Earth Composition of the AIR Water vapour 1 -4% The dry air (by volume) Nitrogen Oxygen Argon Carbon dioxide All others 78 % 21 % 0. 93 % 0. 038 % only trace amounts

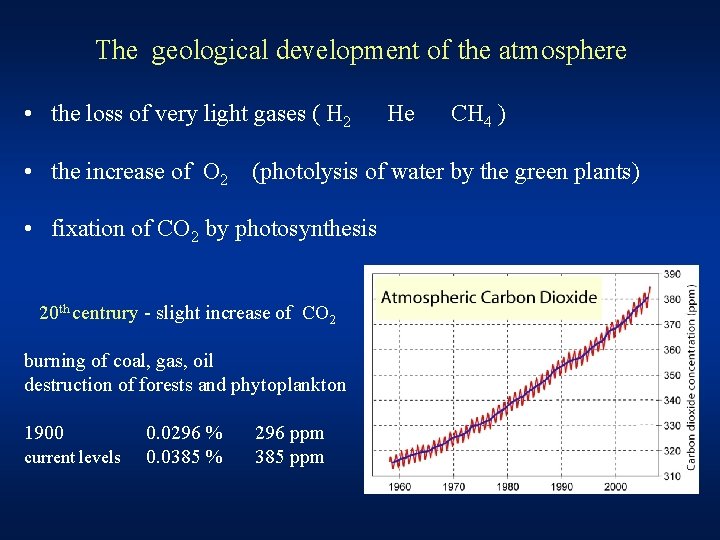
The geological development of the atmosphere • the loss of very light gases ( H 2 He CH 4 ) • the increase of O 2 (photolysis of water by the green plants) • fixation of CO 2 by photosynthesis 20 th centrury - slight increase of CO 2 burning of coal, gas, oil destruction of forests and phytoplankton 1900 current levels 0. 0296 % 0. 0385 % 296 ppm 385 ppm

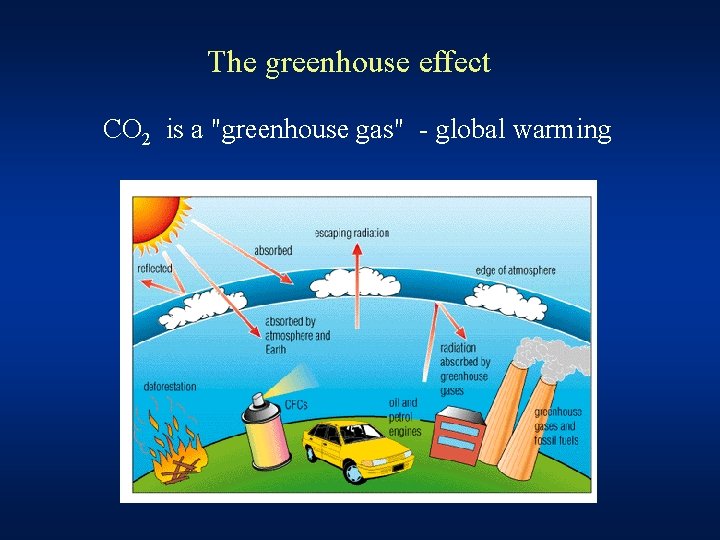
The greenhouse effect CO 2 is a "greenhouse gas" - global warming

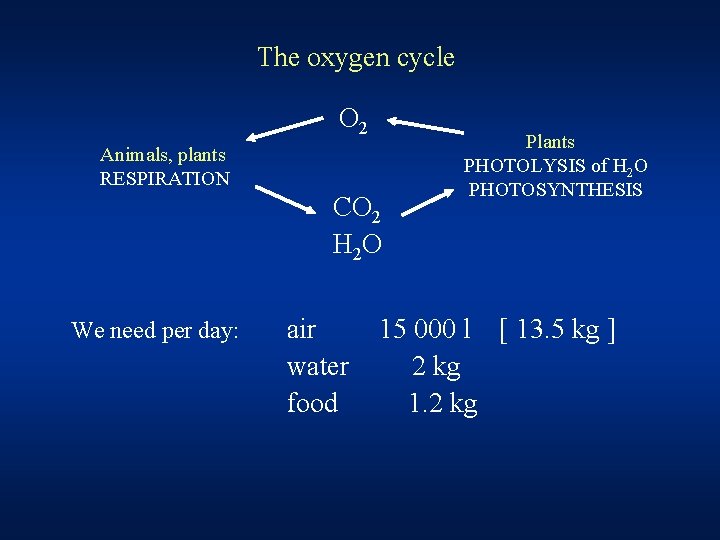
The oxygen cycle O 2 Animals, plants RESPIRATION CO 2 H 2 O We need per day: air water food Plants PHOTOLYSIS of H 2 O PHOTOSYNTHESIS 15 000 l [ 13. 5 kg ] 2 kg 1. 2 kg

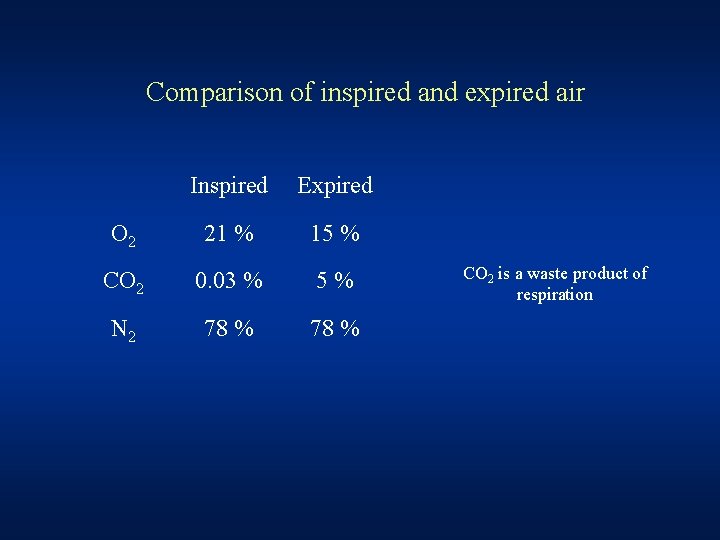
Comparison of inspired and expired air Inspired Expired O 2 21 % 15 % CO 2 0. 03 % 5% N 2 78 % CO 2 is a waste product of respiration

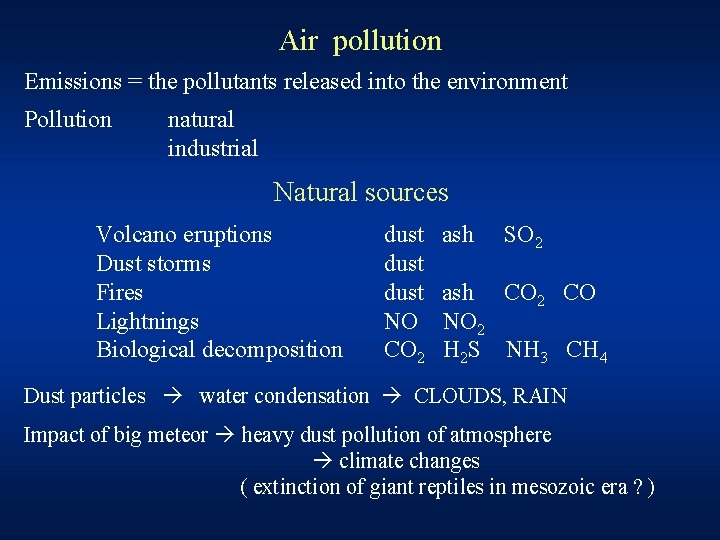
Air pollution Emissions = the pollutants released into the environment Pollution natural industrial Natural sources Volcano eruptions Dust storms Fires Lightnings Biological decomposition dust NO CO 2 ash SO 2 ash CO 2 CO NO 2 H 2 S NH 3 CH 4 Dust particles water condensation CLOUDS, RAIN Impact of big meteor heavy dust pollution of atmosphere climate changes ( extinction of giant reptiles in mesozoic era ? )

Industrial sources The progress of civilization global air pollution (overcharging of the natural cycles of regeneration and detoxication) 1) 2) 3) 4) 5) 6) dust – ashes Sulphur dioxide ( SO 2 ) Nitrogen oxides ( NOx ) Carbon oxides ( CO 2 CO ) Toxic metals Organic compounds

Particulate matter - dust, ashes complex mixture of extremely small particles and liquid droplets sources: power plants, industrial and agriculture processes, transport, home effects: irritation of respiratory system contains adsorbed number of components: toxic metals, SO 2 cancerogenic hydrocarbons the size of particles is directly linked to their potential for causing health problems - smaller than 10 mm pass through the airways and enter the lung ! SMOG [ smoke + fog ] "London smog" HELP: industry, power plants - electrostatic precipitation home - no burning of coal and peat - using gas or oil, solar energy

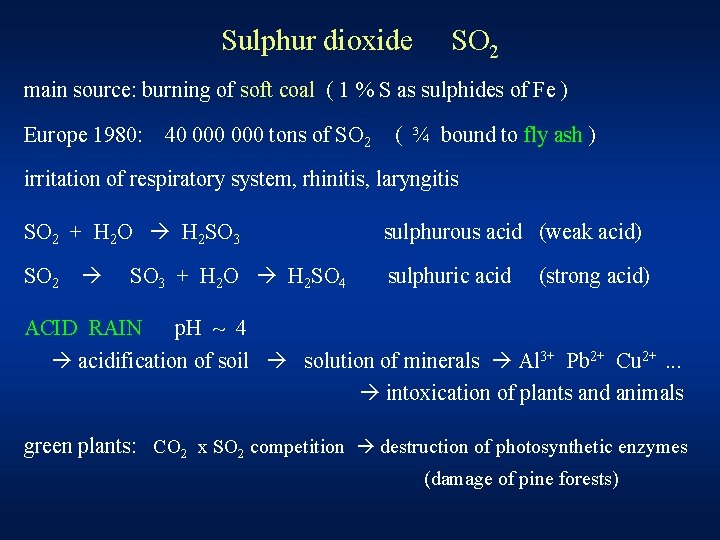
Sulphur dioxide SO 2 main source: burning of soft coal ( 1 % S as sulphides of Fe ) Europe 1980: 40 000 tons of SO 2 ( ¾ bound to fly ash ) irritation of respiratory system, rhinitis, laryngitis SO 2 + H 2 O H 2 SO 3 sulphurous acid (weak acid) SO 2 sulphuric acid SO 3 + H 2 O H 2 SO 4 (strong acid) ACID RAIN p. H ~ 4 acidification of soil solution of minerals Al 3+ Pb 2+ Cu 2+. . . intoxication of plants and animals green plants: CO 2 x SO 2 competition destruction of photosynthetic enzymes (damage of pine forests)

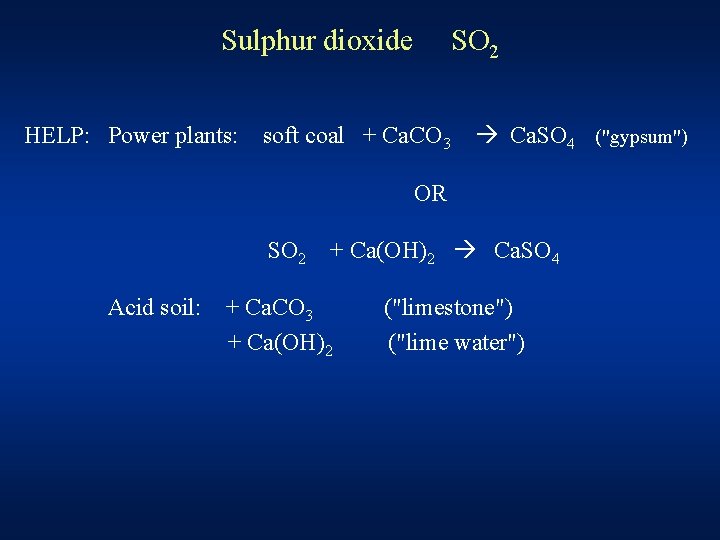
Sulphur dioxide HELP: Power plants: SO 2 soft coal + Ca. CO 3 Ca. SO 4 ("gypsum") OR SO 2 Acid soil: + Ca(OH)2 Ca. SO 4 + Ca. CO 3 + Ca(OH)2 ("limestone") ("lime water")

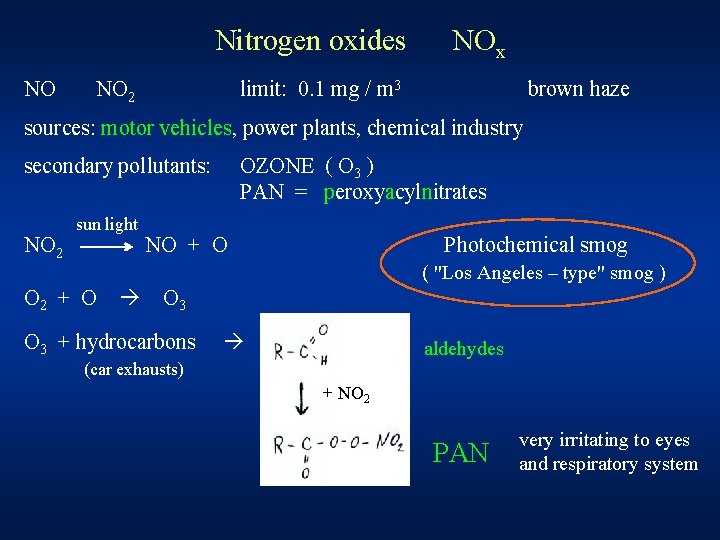
Nitrogen oxides NO NO 2 NOx limit: 0. 1 mg / m 3 brown haze sources: motor vehicles, power plants, chemical industry secondary pollutants: NO 2 sun light OZONE ( O 3 ) PAN = peroxyacylnitrates NO + O Photochemical smog ( "Los Angeles – type" smog ) O 2 + O O 3 + hydrocarbons aldehydes (car exhausts) + NO 2 PAN very irritating to eyes and respiratory system

"London – type" smog combustion of coal = smoke + fog emission of sulphur dioxide ( SO 2 ) and dust + mixed with FOG "Los Angeles – type" smog dry air + sunny days + NOx + volatile organic gases (emitted by automobiles) = photochemical smog

Carbon dioxide CO 2 colorless, odorless gas "overproduction" sources: burning of fossil fuels air: 0. 038 % higher levels stimulate breathing long time respiration of 5 % CO 2 respiration troubles > 15 % DEATH Danger: old mines, caves, wine cellars (heavier than air it tends to go down) Carbon monoxide CO colorless, odorless gas sources: car exhausts, incomplete burning (smouldering) local street pollution – dangerous level: 10 ppm / 8 hours TOXIC: very high affinity to HEMOGLOBIN 35 ppm / 1 hour carbonylhemoglobin impaired ability of the blood to transport O 2 oxygen deprivation drowsiness unconsciousness DEATH

Toxic heavy metals Lead Pb - tetraethyllead was used as antiknock agent in gasoline (organometallic compound) Pb (CH 2 CH 3)4 neurotoxic Pb. O (lead oxide aerosol) deposition in the vicinity of highways toxic effects of inorganic lead compounds: impaired heme synthesis anemia Toxic metals aerosol As, Pb, Cd, Hg, . . . oxides bound to ash particulate matter - source: power plants ( COAL ! ), smelters - general toxicity – decrease of vitality

Organic compounds Hydrocarbons – motor vehicles, industry, cigarette smoke ( TAR ) - carcinogens, precursors to PAN Freons - hydrocarbons with hydrogen atoms substituated by F, Cl = chlorofluorocarbons ( CFCs ) - in older refrigeration and air-conditioning systems - effect: destroying the ozone layer in the stratosphere

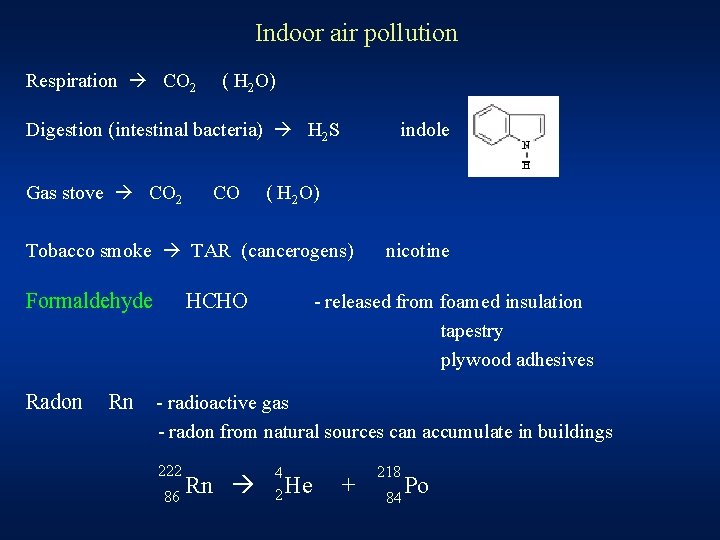
Indoor air pollution Respiration CO 2 ( H 2 O) Digestion (intestinal bacteria) H 2 S Gas stove CO 2 CO indole ( H 2 O) Tobacco smoke TAR (cancerogens) Formaldehyde Radon Rn HCHO nicotine - released from foamed insulation tapestry plywood adhesives - radioactive gas - radon from natural sources can accumulate in buildings 222 86 Rn 4 2 He + 218 84 Po

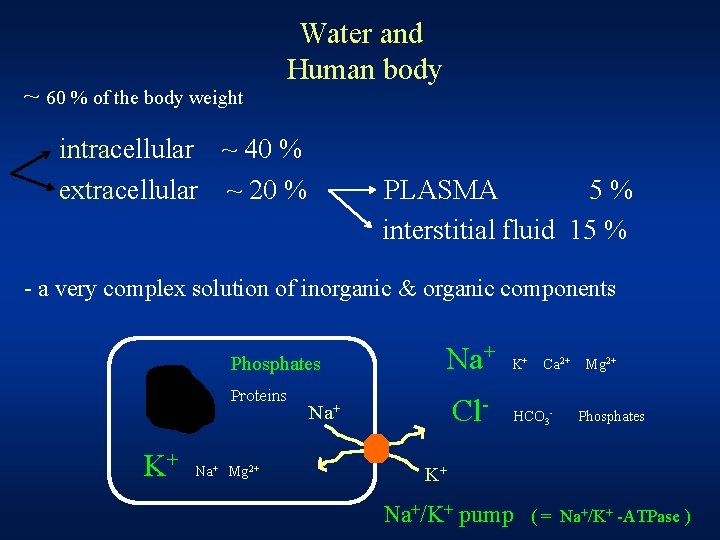
~ 60 % of the body weight Water and Human body intracellular ~ 40 % extracellular ~ 20 % PLASMA 5% interstitial fluid 15 % - a very complex solution of inorganic & organic components Phosphates Proteins K+ Na+ Mg 2+ Na+ Cl- Na+ K+ Ca 2+ HCO 3 - Mg 2+ Phosphates K+ Na+/K+ pump ( = Na+/K+ -ATPase )

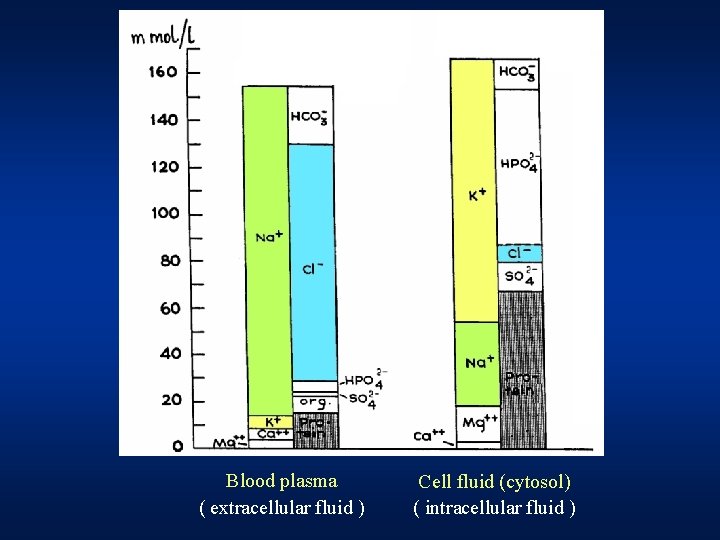
Blood plasma ( extracellular fluid ) Cell fluid (cytosol) ( intracellular fluid )

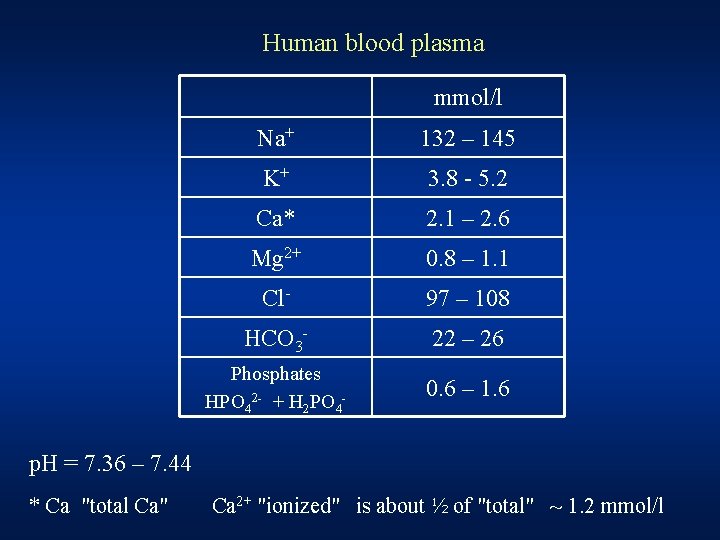
Human blood plasma mmol/l Na+ 132 – 145 K+ 3. 8 - 5. 2 Ca* 2. 1 – 2. 6 Mg 2+ 0. 8 – 1. 1 Cl- 97 – 108 HCO 3 - 22 – 26 Phosphates HPO 42 - + H 2 PO 4 - 0. 6 – 1. 6 p. H = 7. 36 – 7. 44 * Ca "total Ca" Ca 2+ "ionized" is about ½ of "total" ~ 1. 2 mmol/l

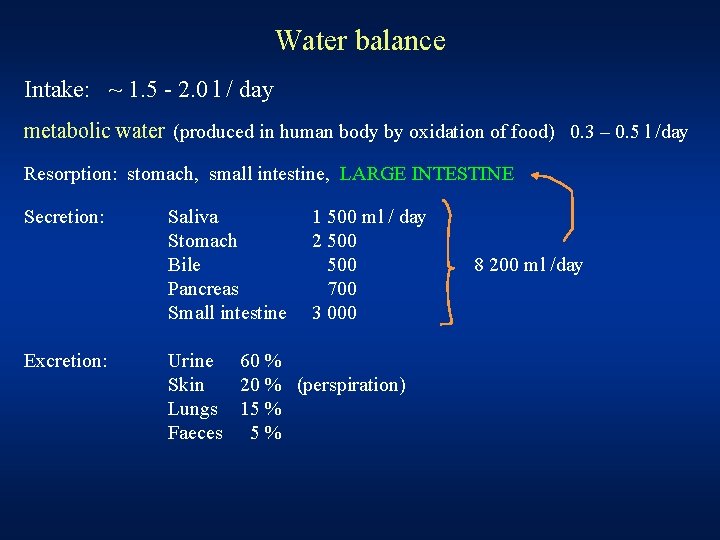
Water balance Intake: ~ 1. 5 - 2. 0 l / day metabolic water (produced in human body by oxidation of food) 0. 3 – 0. 5 l /day Resorption: stomach, small intestine, LARGE INTESTINE Secretion: Excretion: Saliva Stomach Bile Pancreas Small intestine 1 500 ml / day 2 500 700 3 000 Urine 60 % Skin 20 % (perspiration) Lungs 15 % Faeces 5 % 8 200 ml /day

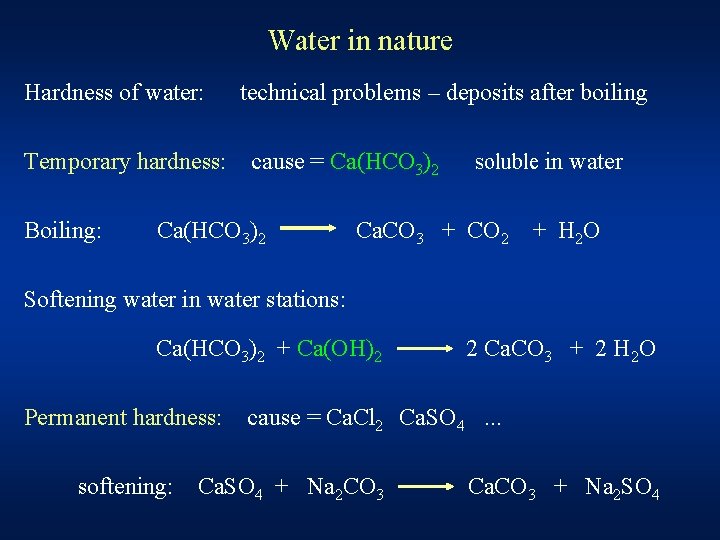
Water in nature Hardness of water: Temporary hardness: Boiling: technical problems – deposits after boiling cause = Ca(HCO 3)2 soluble in water Ca. CO 3 + CO 2 + H 2 O Softening water in water stations: Ca(HCO 3)2 + Ca(OH)2 Permanent hardness: softening: 2 Ca. CO 3 + 2 H 2 O cause = Ca. Cl 2 Ca. SO 4. . . Ca. SO 4 + Na 2 CO 3 Ca. CO 3 + Na 2 SO 4

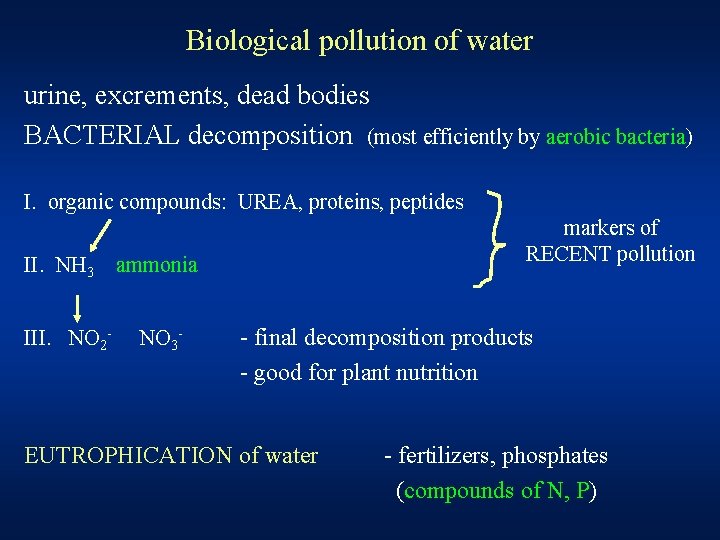
Biological pollution of water urine, excrements, dead bodies BACTERIAL decomposition (most efficiently by aerobic bacteria) I. organic compounds: UREA, proteins, peptides II. NH 3 III. NO 2 - markers of RECENT pollution ammonia NO 3 - - final decomposition products - good for plant nutrition EUTROPHICATION of water - fertilizers, phosphates (compounds of N, P)

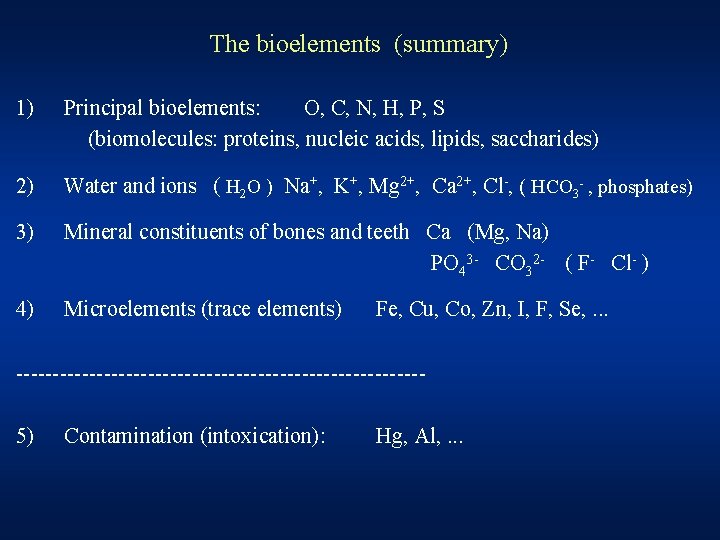
The bioelements (summary) 1) Principal bioelements: O, C, N, H, P, S (biomolecules: proteins, nucleic acids, lipids, saccharides) 2) Water and ions ( H 2 O ) Na+, K+, Mg 2+, Ca 2+, Cl-, ( HCO 3 - , phosphates) 3) Mineral constituents of bones and teeth Ca (Mg, Na) PO 43 - CO 32 - ( F- Cl- ) 4) Microelements (trace elements) Fe, Cu, Co, Zn, I, F, Se, . . . ----------------------------5) Contamination (intoxication): Hg, Al, . . .