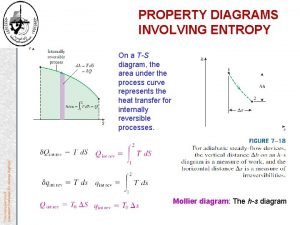
Laws of Thermodynamics Heat Engines and Entropy First


- Slides: 8

Laws of Thermodynamics Heat Engines and Entropy

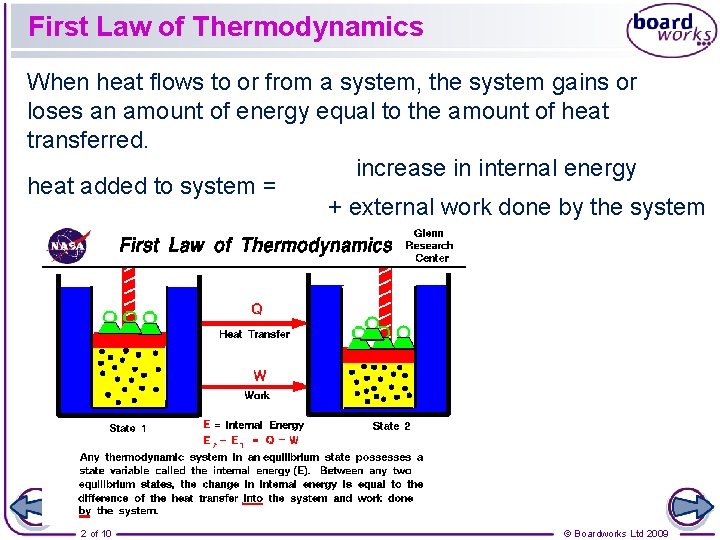
First Law of Thermodynamics When heat flows to or from a system, the system gains or loses an amount of energy equal to the amount of heat transferred. increase in internal energy heat added to system = + external work done by the system 2 of 10 © Boardworks Ltd 2009

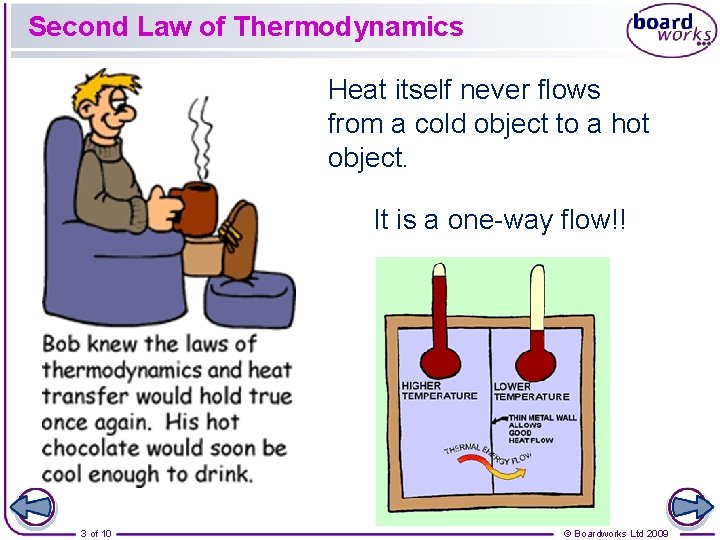
Second Law of Thermodynamics Heat itself never flows from a cold object to a hot object. It is a one-way flow!! 3 of 10 © Boardworks Ltd 2009

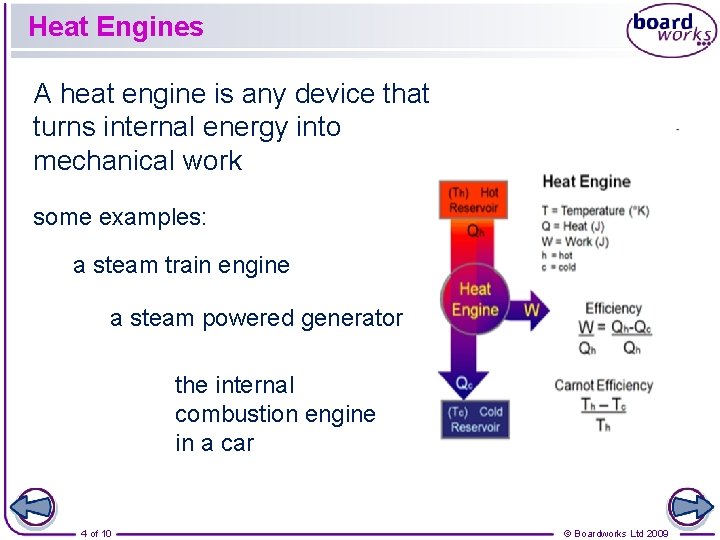
Heat Engines A heat engine is any device that turns internal energy into mechanical work some examples: a steam train engine a steam powered generator the internal combustion engine in a car 4 of 10 © Boardworks Ltd 2009

Not all the heat gets used for work A French physicist named Sadi Carnot figured out that the greatest efficiency (how much work you get for the heat put in) comes with the greatest difference in temperature. However no heat engine is 100% efficient, some heat gets "lost" to entropy 5 of 10 © Boardworks Ltd 2009

Entropy high quality (useful) energy tends to turn into low quality energy (not useful) or order tends to turn into disorder. Your bedroom is a great example of this. You work hard to clean it and soon it is messy again. 6 of 10 © Boardworks Ltd 2009

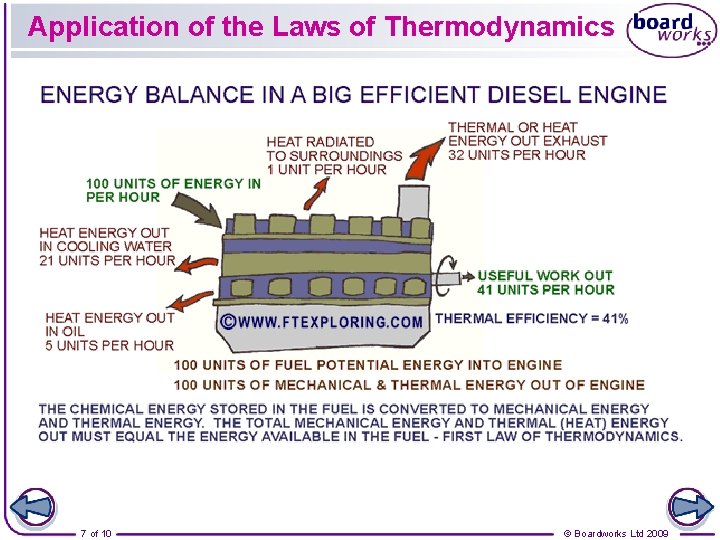
Application of the Laws of Thermodynamics 7 of 10 © Boardworks Ltd 2009

Check for understanding – true or false or give a number. 1. Cold energy flows from the cold object to the hot object FALSE 2. It takes work (the transfer of energy) to keep your room orderly. TRUE 3. Heat energy can not be easily recycled into work. TRUE 4. The diesel engine only does 41 units of useful work for 100 units energy in it fuel. How much energy is “lost” to friction and entropy? 59 units of energy 5. A heat engine does 60 J of work for every 100 0. 6 or J of heat energy input into the engine. What is 60% the engine’s efficiency? efficient 8 of 10 © Boardworks Ltd 2009