Law of Conservation of Mass The law of



- Slides: 17

Law of Conservation of Mass The law of conservation of mass explains that matter cannot be created or destroyed, it can only change forms. Therefore, we cannot destroy something. All we can do is change the form that it exists as. Was our paper destroyed?

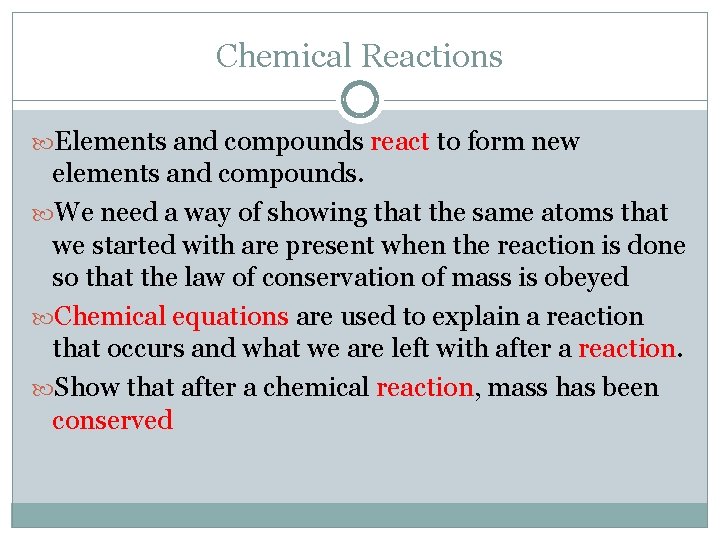
Chemical Reactions Elements and compounds react to form new elements and compounds. We need a way of showing that the same atoms that we started with are present when the reaction is done so that the law of conservation of mass is obeyed Chemical equations are used to explain a reaction that occurs and what we are left with after a reaction. Show that after a chemical reaction, mass has been conserved

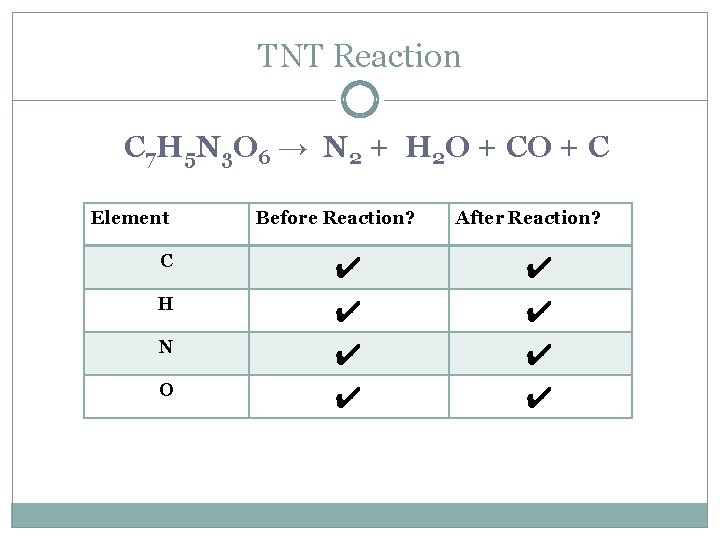
TNT Reaction C 7 H 5 N 3 O 6 → N 2 + H 2 O + C Element C H N O Before Reaction? ✔ ✔ After Reaction? ✔ ✔

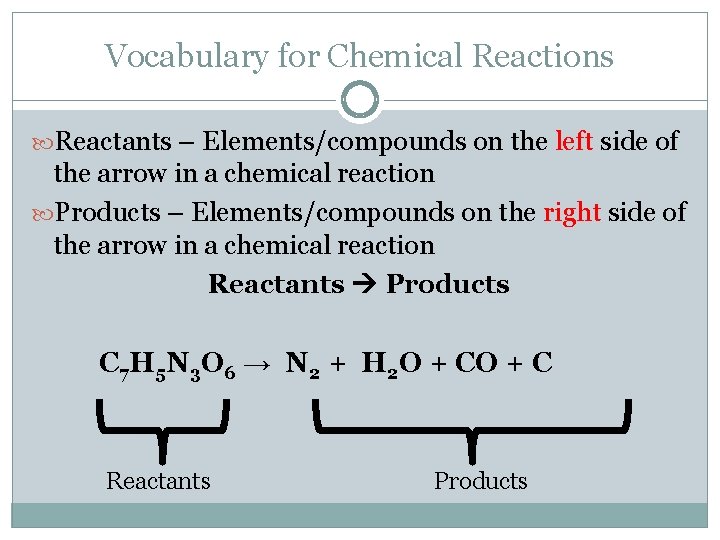
Vocabulary for Chemical Reactions Reactants – Elements/compounds on the left side of the arrow in a chemical reaction Products – Elements/compounds on the right side of the arrow in a chemical reaction Reactants Products C 7 H 5 N 3 O 6 → N 2 + H 2 O + C Reactants Products

States of Molecules We describe the state that a elements/compounds exist in during a chemical reactions. A molecule can be in a solid, liquid, gas, or aqueous state. Aqueous means that the element/compound is dissolved in water. We abbreviate this as follows: (s) = solid (l) = liquid (g) = gas (aq) = aqueous

Table Talk With your table, label the products, the reactants, and the state of each compound. NH 4 OH (aq) + Fe. Cl 3 (s) NH 4 Cl (s) + Fe(OH) 3 (aq)

Stop and Jot For the following reaction, label the products, the reactants, and the state of each compound: H 2 O 2 (aq) + Na. Br (s) Na. OH (aq) + H 2 O (l) + O 2 (g)

Essential Point MOST CHEMICAL REACTIONS, AS WRITTEN, DO NOT SATISFY THE LAW OF CONSERVATION OF MASS. THERE ARE MORE ATOMS ON ONE SIDE THEN ON THE OTHER.

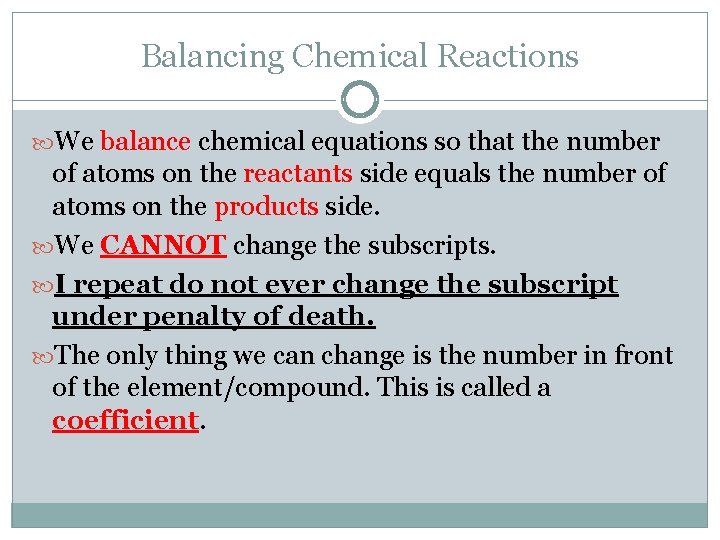
Balancing Chemical Reactions Since the law of conservation of mass states that we cannot create/destroy matter, we need to have the same amount on each side of an equation. Therefore, we need the same amount of atoms on each side of an equation to satisfy this law. We need to fix our chemical equations so that we can properly obey this law.

Balancing Chemical Reactions We balance chemical equations so that the number of atoms on the reactants side equals the number of atoms on the products side. We CANNOT change the subscripts. I repeat do not ever change the subscript under penalty of death. The only thing we can change is the number in front of the element/compound. This is called a coefficient.

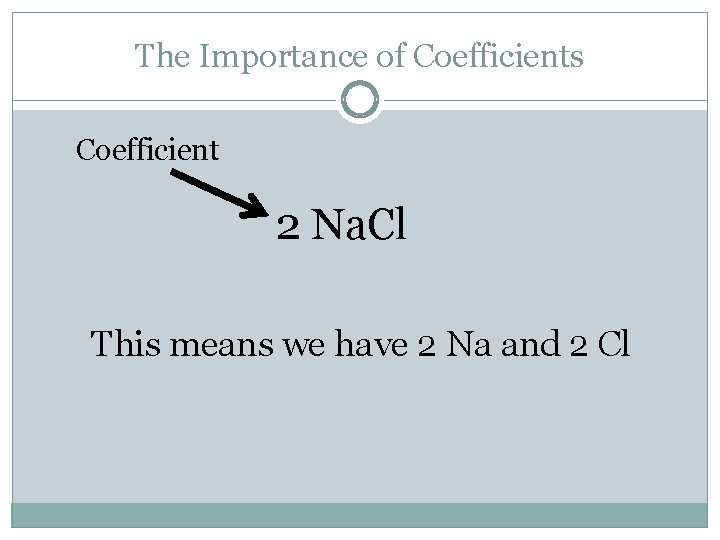
The Importance of Coefficients Coefficient 2 Na. Cl This means we have 2 Na and 2 Cl

Organizing Our Thoughts Element/Co # Atoms in mpound Reactants # of Atoms in Products Balanced?

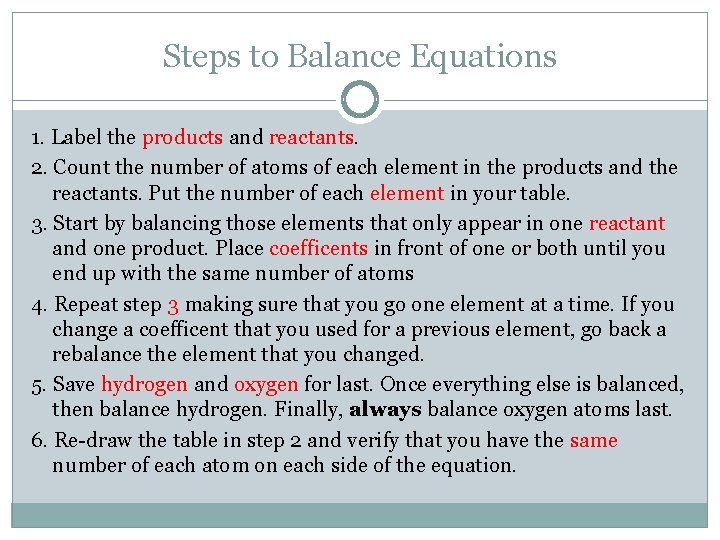
Steps to Balance Equations 1. Label the products and reactants. 2. Count the number of atoms of each element in the products and the reactants. Put the number of each element in your table. 3. Start by balancing those elements that only appear in one reactant and one product. Place coefficents in front of one or both until you end up with the same number of atoms 4. Repeat step 3 making sure that you go one element at a time. If you change a coefficent that you used for a previous element, go back a rebalance the element that you changed. 5. Save hydrogen and oxygen for last. Once everything else is balanced, then balance hydrogen. Finally, always balance oxygen atoms last. 6. Re-draw the table in step 2 and verify that you have the same number of each atom on each side of the equation.

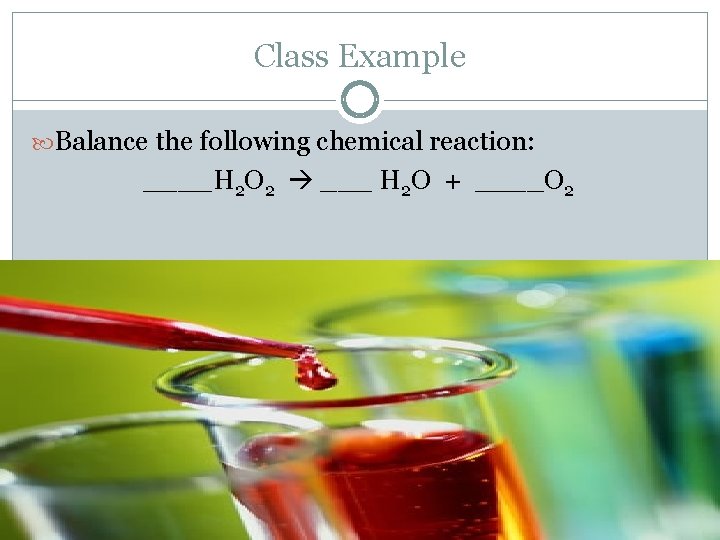
Class Example Balance the following chemical reaction: ____H 2 O 2 ___ H 2 O + ____O 2

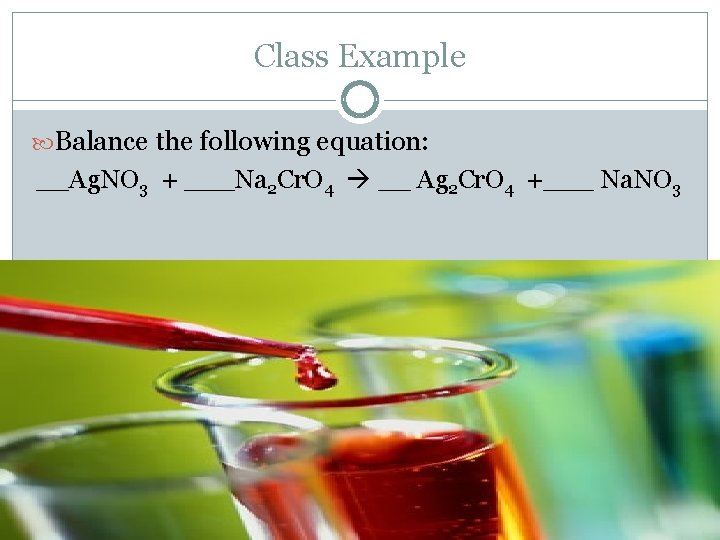
Class Example Balance the following equation: __Ag. NO 3 + ___Na 2 Cr. O 4 __ Ag 2 Cr. O 4 +___ Na. NO 3

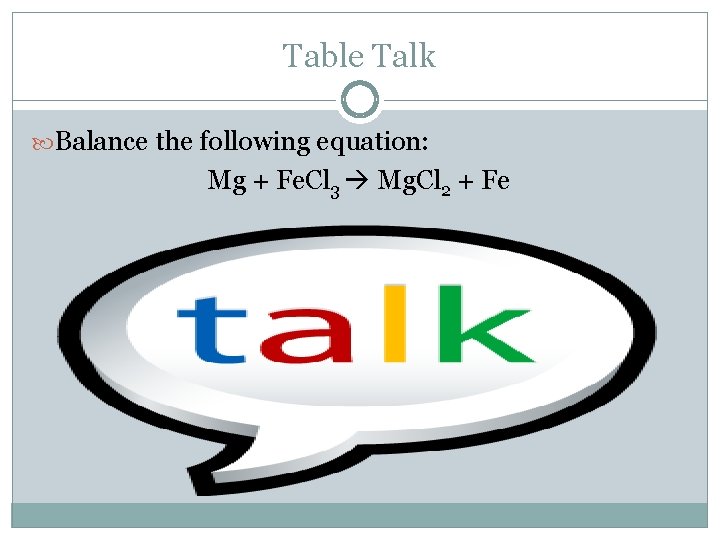
Table Talk Balance the following equation: Mg + Fe. Cl 3 Mg. Cl 2 + Fe

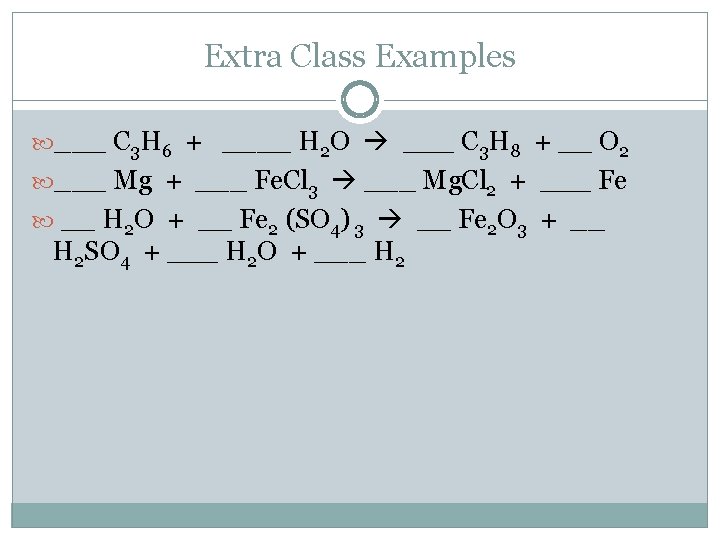
Extra Class Examples ___ C 3 H 6 + ____ H 2 O ___ C 3 H 8 + __ O 2 ___ Mg + ___ Fe. Cl 3 ___ Mg. Cl 2 + ___ Fe __ H 2 O + __ Fe 2 (SO 4) 3 __ Fe 2 O 3 + __ H 2 SO 4 + ___ H 2 O + ___ H 2
 Antoine lavoisier law
Antoine lavoisier law Law of conservation od mass
Law of conservation od mass Law of conservation of mass lab worksheet answers
Law of conservation of mass lab worksheet answers Law of conservation of mass
Law of conservation of mass Law of conservation of mass
Law of conservation of mass Law of conservation of mass
Law of conservation of mass Control volume approach
Control volume approach Law of conservation of mass
Law of conservation of mass Br3cl9 name
Br3cl9 name Conservation of mass examples
Conservation of mass examples Law of conservation of mass examples
Law of conservation of mass examples Law of conservation of mass
Law of conservation of mass Conservation of mass
Conservation of mass Example of law of conservation of mass
Example of law of conservation of mass Equação da continuidade
Equação da continuidade Mass conservation equation
Mass conservation equation Momentum conservation fluid mechanics
Momentum conservation fluid mechanics Conservation of mass momentum and energy equations
Conservation of mass momentum and energy equations