l l l l 1 1 Inductively Coupled
















- Slides: 23





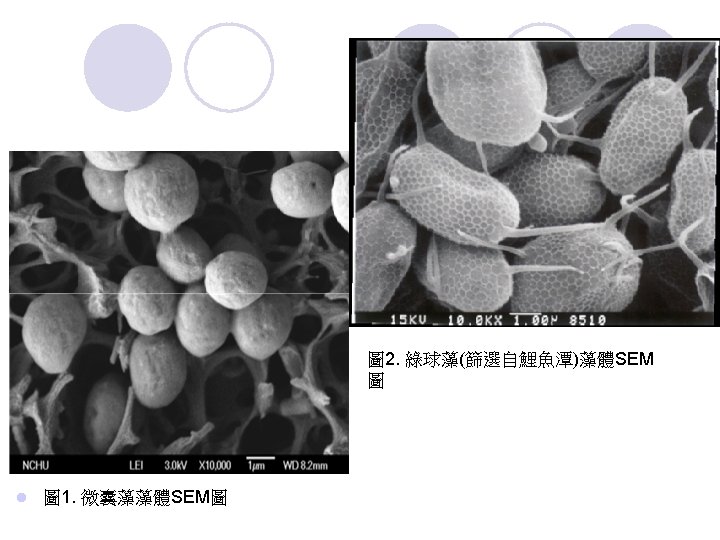
二、研究方法 l l l l 1. 實驗材料、設備及藥品 (1) 感應耦合電漿質譜儀(Inductively Coupled Plasma Mass Spectrometry,ICP-MS) (Perkin Elmer Sciex ELAN DRCⅡ) (2) 旋轉攪拌器(Kansin Instruments Co. , Ltd. , Taiwan) (3) 場發射掃描電子顯微鏡(Field-emission scanning electron microscope,FESEM) (JEOL JSM-6700 F Oxford inca energy 400) (4) 湯瑪氏血球計數盤(Thomas haemacytometer)(Neubauer Improved Bright-Line Marienfeld,Germany) (5) 螢光顯微鏡(Nikon Eclipse E 600) (6) 微波消化(MARS-5, CEM) (7) 高效能液相層析儀(High Performance Liquid Chromatograph , HPLC)( Perkin Elmer Series 200 pump)

l l l l (8) 汞標準品(Mercury standard solution):Mercury (II) nitrate in nitric acid 0. 5 mol/L(Merck,Germany) (9) 甲基汞標準品(Methylmercury-chloride):purity≧ 95. 5%(Dr. Ehrenstorfer,Merck,Germany) (10) Na. HCO 3(Sodium Bicarbonate) purity≧ 99%(GR 級,OSAKA, Japan) (11) Na 3 C 6 H 5 O 7.2 H 2 O(Sodium Citrate):purity≧ 99%(GR 級, OSAKA,Japan) (12) Na 2 S 2 O 4(Sodium Hydrosulfite):purity≧ 99%(GR 級,OSAKA, Japan) (13) 低汞硝酸(GR 級,E-Merck,Germany) (14) 戊二醛(Fluka,USA)

















五、 參考文獻 l l l l l 1. Biester, H. , Schuhmacher, P. , Müller, G. , “Effectiveness of mossy tin filters to remove mercury from aqueous solution by Hg(II) reduction and Hg(0) amalgamation, ” Water Research, Vol. 34, Iss. 7, pp. 2031 -2036 (2000). 2. Paraquetti, H. H. M. , Ayres, G. A. , Almeida, M. D. de, Molisani, M. M. and Lacerda, L. D. de, “Mercury distribution, speciation and flux in the Sepetiba Bay tributaries, SE Brazil, ” Water Research, Vol. 38, Iss. 6, pp. 1439 -1448 (2004). 3. He, T. , Feng, X. , Guo, Y. , Qiu, G. , Li, Z. , Liang, L. and Lu, J. , “The impact of eutrophication on the biogeochemical cycling of mercury species in a reservoir: A case study from Hongfeng Reservoir, Guizhou, China, ” Environmental Pollution, Vol. 154, Iss. 1, pp. 56 -67 (2008). 4. Awasthi, M. and Rai, L. C. , “Adsorption of nickel, zinc and cadmium byimmobilized green algae and cyanobacteria: a comparative study, ” Annals of microbiology, Vol. 54, No. 3, pp. 257 -267 (2004). 5. Struck, B. D. , Pelzer, R. , Ostapczuk P. , Emons H. and Mohl C. , “Statistical evaluation of ecosystem properties influencing the uptake of As, Cd, Co, Cu, Hg, Mn, Ni, Pb and Zn in seaweed (Fucus vesiculosus) and common mussel (Mytilusedulis), ” The Science of The Total Environment, Vol. 207, Iss. 1, pp. 29 -42 (1997). 6. Csonto, J. , Kadukova, J. and Polak, M. , “Artificial life simulation of living alga cells and its sorption mechanisms, ” Journal of Medical Systems, Vol. 25, Iss. 3, pp. 221 -231 ( 2001). 7. Rai, L. C. , Gaur J. P. and Soeder C. J. (ed. ), “Algae and Water Pollution, ” E. Schweizerbart’sche Verlagsbuchhandlung science, Advances in Limnology, Germany, Vol. 42, pp. 1 -29 (1994). 8. USEPA (U. S. Environmental Protection Agency), ”Mercury Study Report to Congress, ” Vol. 1, Executive Summary, EPA-452/R-97 -003, (Washington: GPO) (1997). 9. Taylor GJ, Crowder A. A. “Use of the DCB technique for extraction of hydrous ironoxides from roots of wetland plants, ” Amer. J Bot. ; 70, pp. 1254– 1257. 23 (1983).