Chapter 15 Molecular Luminescence Spectrometry Features inherent sensitivity

![FIGURE 15 -6(b) In (b), the total fluorescence spectrum of 8 hydroxybenzo[a]pyrene is shown FIGURE 15 -6(b) In (b), the total fluorescence spectrum of 8 hydroxybenzo[a]pyrene is shown](https://slidetodoc.com/presentation_image_h/2ce5cc17639a95ed5ea1db0eec18cb30/image-17.jpg)


- Slides: 33

Chapter 15 Molecular Luminescence Spectrometry Features: inherent sensitivity, large linear concentration ranges. Photoluminescence (光激發光): (1) Fluorescence (2) Phosphorescence Chemiluminescence (化學發光)

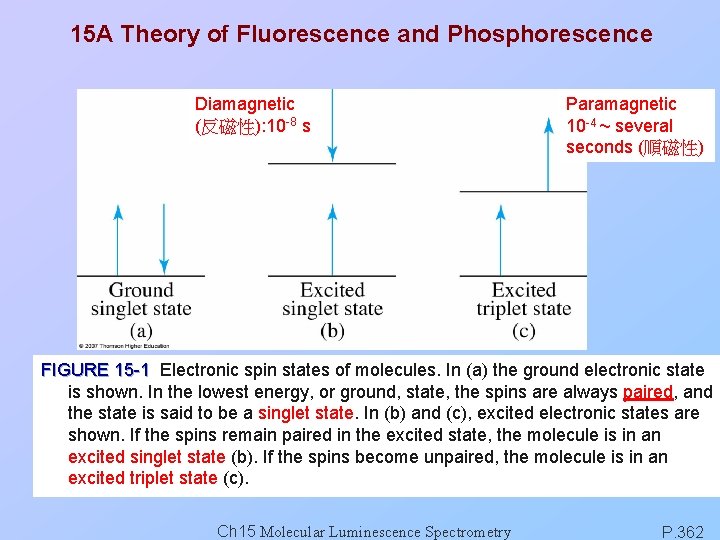
15 A Theory of Fluorescence and Phosphorescence Diamagnetic (反磁性): 10 -8 s Paramagnetic 10 -4 ~ several seconds (順磁性) FIGURE 15 -1 Electronic spin states of molecules. In (a) the ground electronic state is shown. In the lowest energy, or ground, state, the spins are always paired, and the state is said to be a singlet state. In (b) and (c), excited electronic states are shown. If the spins remain paired in the excited state, the molecule is in an excited singlet state (b). If the spins become unpaired, the molecule is in an excited triplet state (c). Ch 15 Molecular Luminescence Spectrometry P. 362

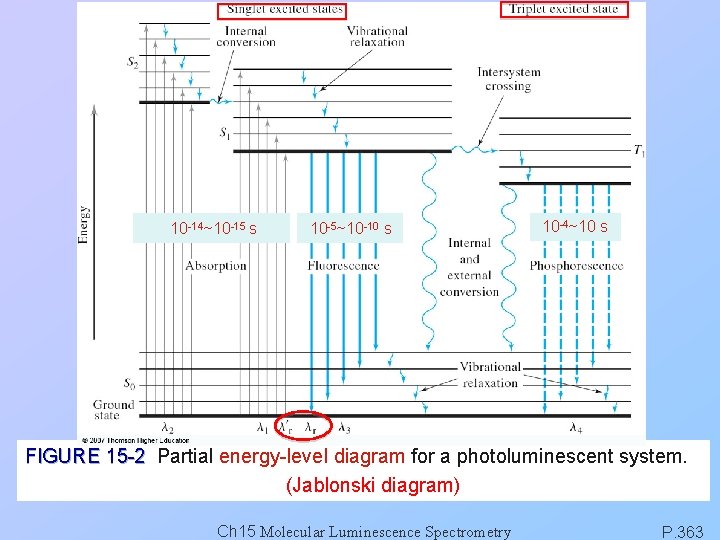
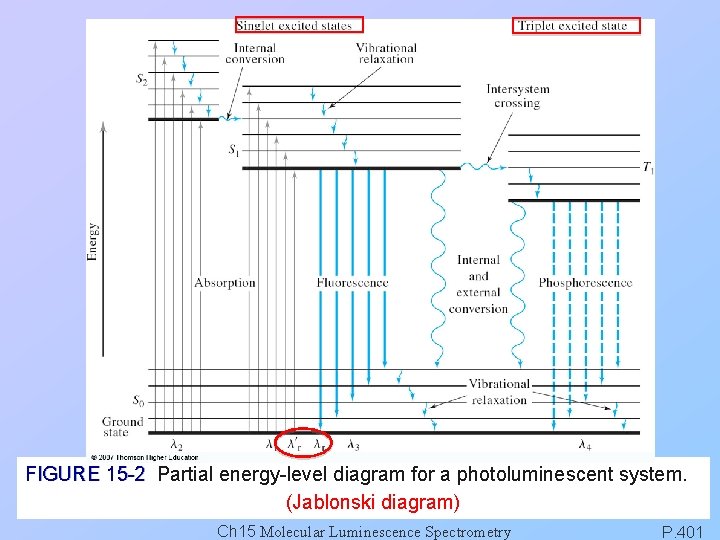
10 -14~10 -15 s 10 -5~10 -10 s 10 -4~10 s FIGURE 15 -2 Partial energy-level diagram for a photoluminescent system. (Jablonski diagram) Ch 15 Molecular Luminescence Spectrometry P. 363

Deactivation processes • • Vibrational deactivation (<10 -12 s) Internal conversion predissiociation dissociation (x) • External conversion • Intersystem crossing (S 1 to T 1) • Radiationless processes (heavy atom effect) Fluorescence • Phosphorescence • Radiation processes

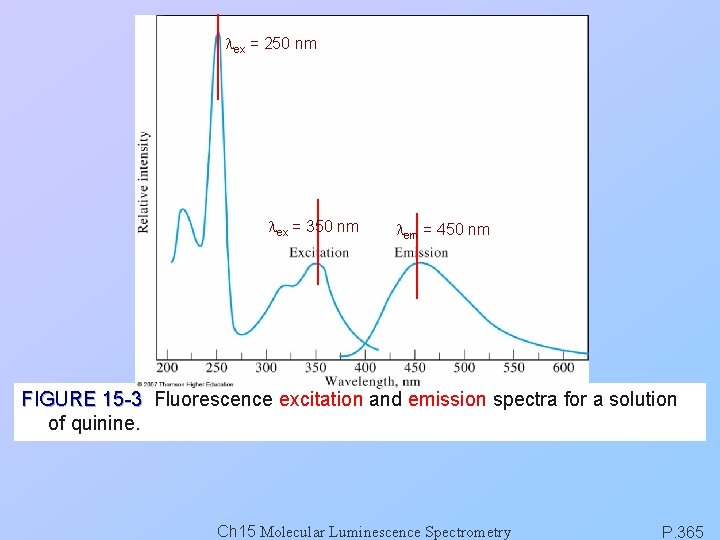
lex = 250 nm lex = 350 nm lem = 450 nm FIGURE 15 -3 Fluorescence excitation and emission spectra for a solution of quinine. Ch 15 Molecular Luminescence Spectrometry P. 365

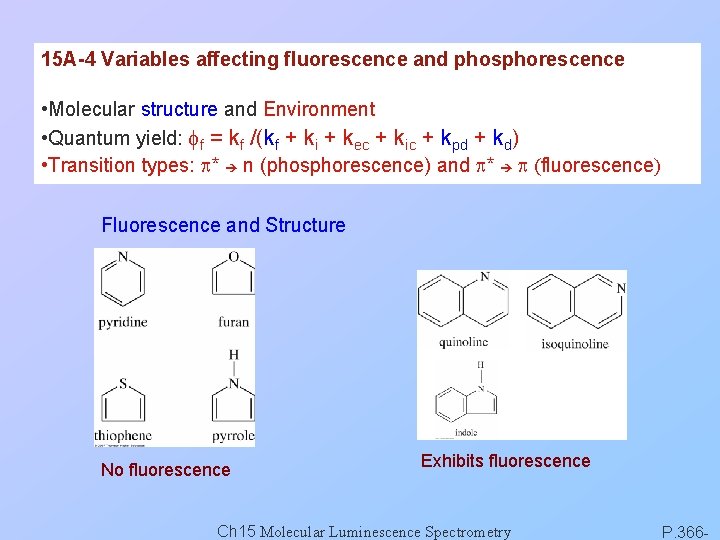
15 A-4 Variables affecting fluorescence and phosphorescence • Molecular structure and Environment • Quantum yield: ff = kf /(kf + ki + kec + kic + kpd + kd) • Transition types: p* n (phosphorescence) and p* p (fluorescence) Fluorescence and Structure No fluorescence Exhibits fluorescence Ch 15 Molecular Luminescence Spectrometry P. 366 -

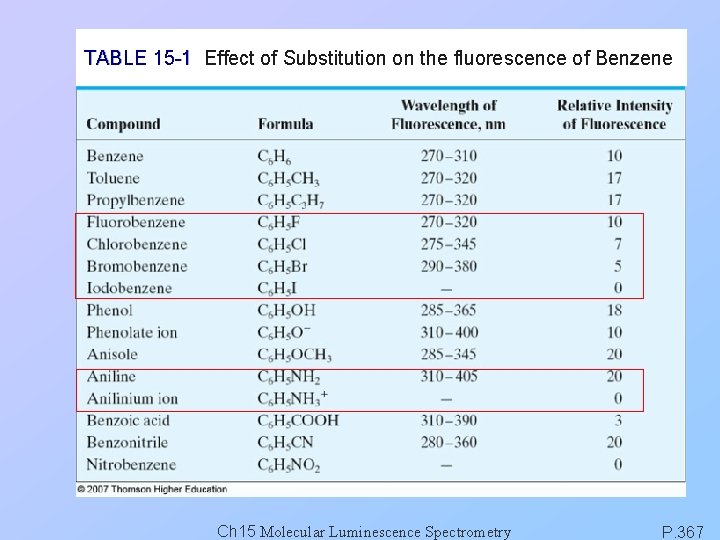
TABLE 15 -1 Effect of Substitution on the fluorescence of Benzene Ch 15 Molecular Luminescence Spectrometry P. 367

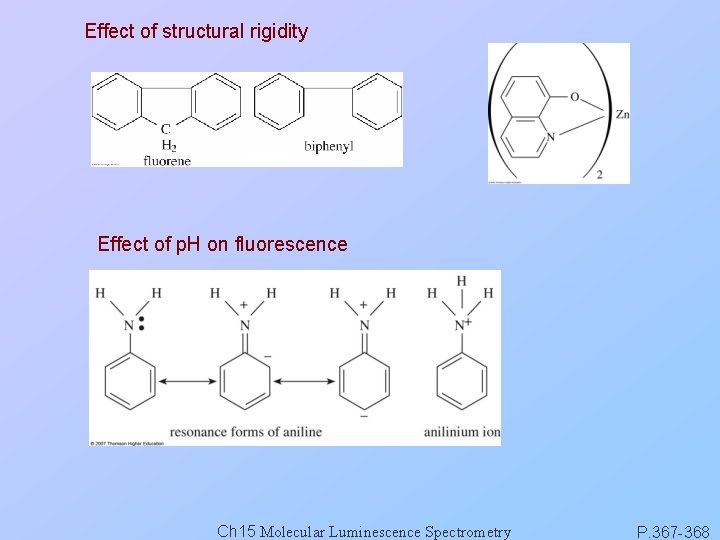
Effect of structural rigidity Effect of p. H on fluorescence Ch 15 Molecular Luminescence Spectrometry P. 367 -368

Review 1) 2) 3) Describe all possible relaxation processes of a singlet excited molecule. Why Stokes shift is constantly observed in molecular spectra? How about atomic spectra? What is the heavy-atom effect in fluorescence?

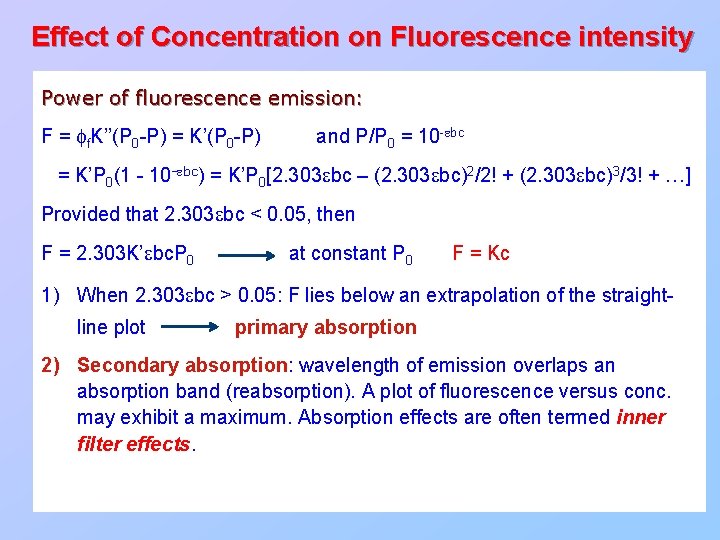
Effect of Concentration on Fluorescence intensity Power of fluorescence emission: F = ff. K’’(P 0 -P) = K’(P 0 -P) and P/P 0 = 10 -ebc = K’P 0(1 - 10 -ebc) = K’P 0[2. 303 ebc – (2. 303 ebc)2/2! + (2. 303 ebc)3/3! + …] Provided that 2. 303 ebc < 0. 05, then F = 2. 303 K’ebc. P 0 at constant P 0 F = Kc 1) When 2. 303 ebc > 0. 05: F lies below an extrapolation of the straightline plot primary absorption 2) Secondary absorption: wavelength of emission overlaps an absorption band (reabsorption). A plot of fluorescence versus conc. may exhibit a maximum. Absorption effects are often termed inner filter effects.

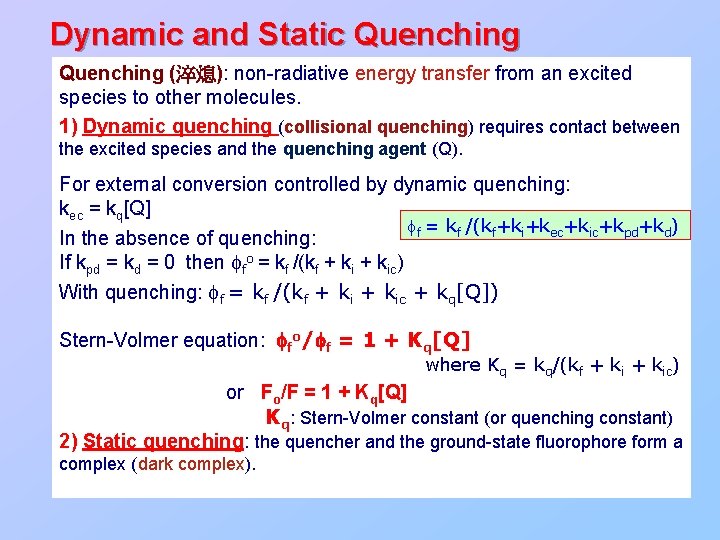
Dynamic and Static Quenching (淬熄): non-radiative energy transfer from an excited species to other molecules. 1) Dynamic quenching (collisional quenching) requires contact between the excited species and the quenching agent (Q). For external conversion controlled by dynamic quenching: kec = kq[Q] ff = kf /(kf+ki+kec+kic+kpd+kd) In the absence of quenching: If kpd = kd = 0 then ffo = kf /(kf + kic) With quenching: ff = kf /(kf + kic + kq[Q]) Stern-Volmer equation: ffo/ff = 1 + Kq[Q] where Kq = kq/(kf + kic) or Fo/F = 1 + Kq[Q] Kq: Stern-Volmer constant (or quenching constant) 2) Static quenching: the quencher and the ground-state fluorophore form a complex (dark complex).

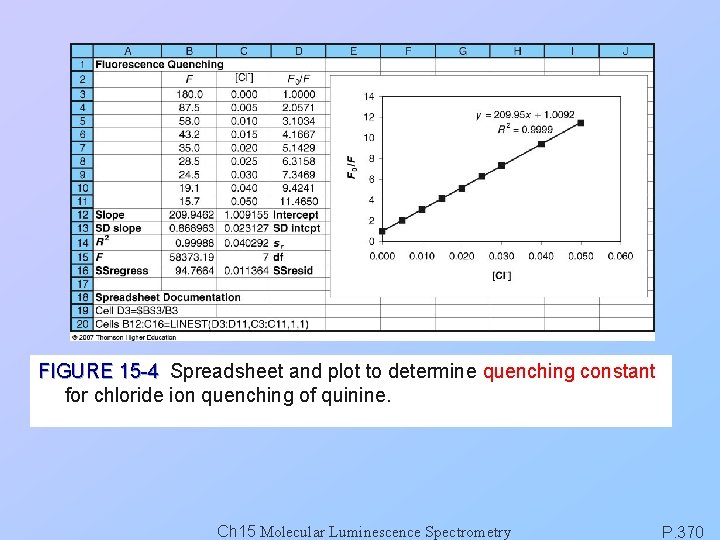
FIGURE 15 -4 Spreadsheet and plot to determine quenching constant for chloride ion quenching of quinine. Ch 15 Molecular Luminescence Spectrometry P. 370

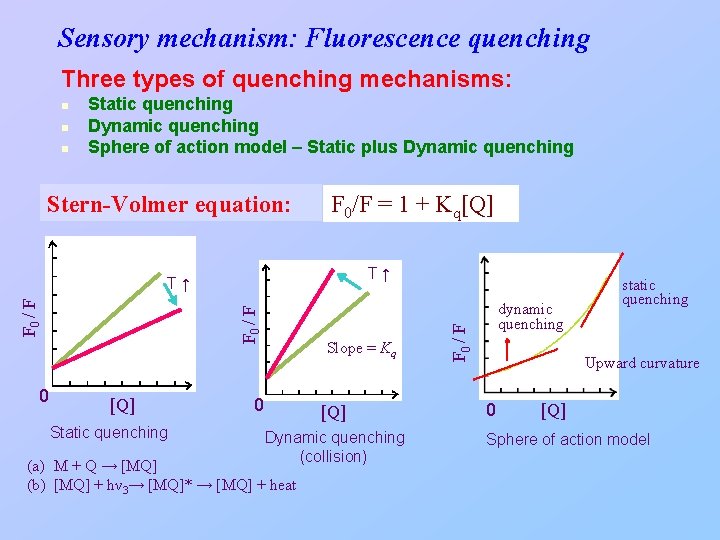
Sensory mechanism: Fluorescence quenching Three types of quenching mechanisms: n n Static quenching Dynamic quenching Sphere of action model – Static plus Dynamic quenching Stern-Volmer equation: F 0/F = 1 + Kq[Q] T↑ 0 F 0 / F T↑ [Q] Static quenching Slope = Kq 0 [Q] Dynamic quenching (collision) (a) M + Q → [MQ] (b) [MQ] + hν 3→ [MQ]* → [MQ] + heat dynamic quenching F 0 / F n static quenching Upward curvature 0 [Q] Sphere of action model

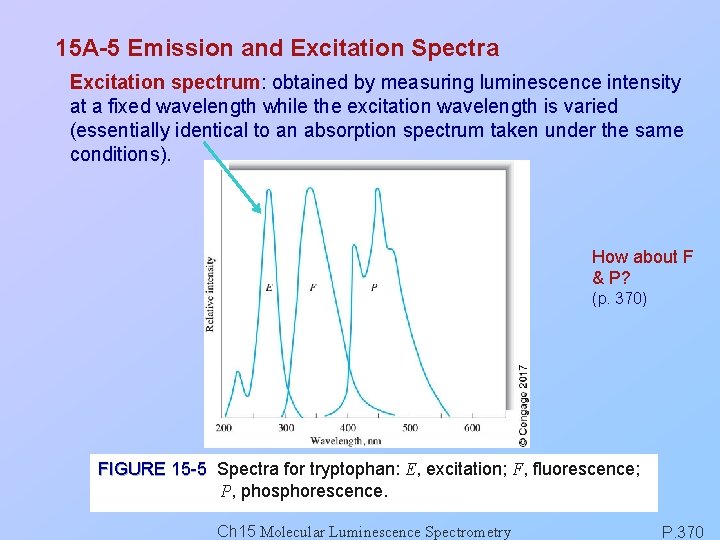
15 A-5 Emission and Excitation Spectra Excitation spectrum: obtained by measuring luminescence intensity at a fixed wavelength while the excitation wavelength is varied (essentially identical to an absorption spectrum taken under the same conditions). How about F & P? (p. 370) FIGURE 15 -5 Spectra for tryptophan: E, excitation; F, fluorescence; P, phosphorescence. Ch 15 Molecular Luminescence Spectrometry P. 370

FIGURE 15 -2 Partial energy-level diagram for a photoluminescent system. (Jablonski diagram) Ch 15 Molecular Luminescence Spectrometry P. 401

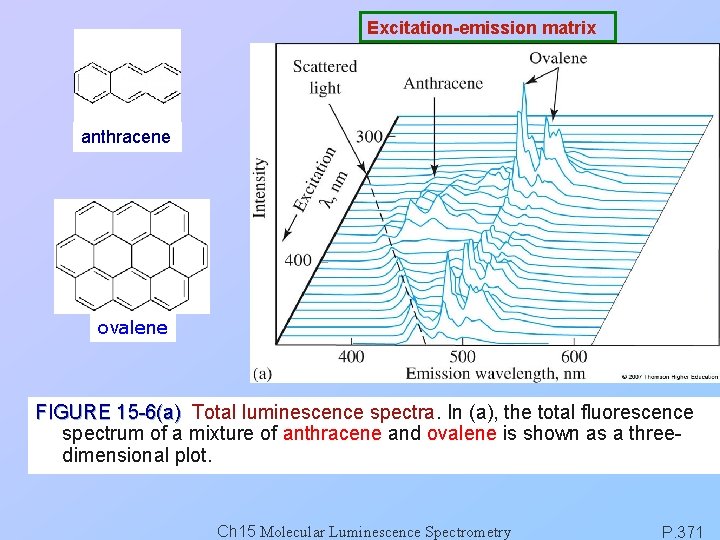
Excitation-emission matrix anthracene ovalene FIGURE 15 -6(a) Total luminescence spectra. In (a), the total fluorescence spectrum of a mixture of anthracene and ovalene is shown as a threedimensional plot. Ch 15 Molecular Luminescence Spectrometry P. 371
![FIGURE 15 6b In b the total fluorescence spectrum of 8 hydroxybenzoapyrene is shown FIGURE 15 -6(b) In (b), the total fluorescence spectrum of 8 hydroxybenzo[a]pyrene is shown](https://slidetodoc.com/presentation_image_h/2ce5cc17639a95ed5ea1db0eec18cb30/image-17.jpg)
FIGURE 15 -6(b) In (b), the total fluorescence spectrum of 8 hydroxybenzo[a]pyrene is shown as a contour plot (等高線圖). Each line represents a particular fluorescence intensity. Ch 15 Molecular Luminescence Spectrometry P. 371

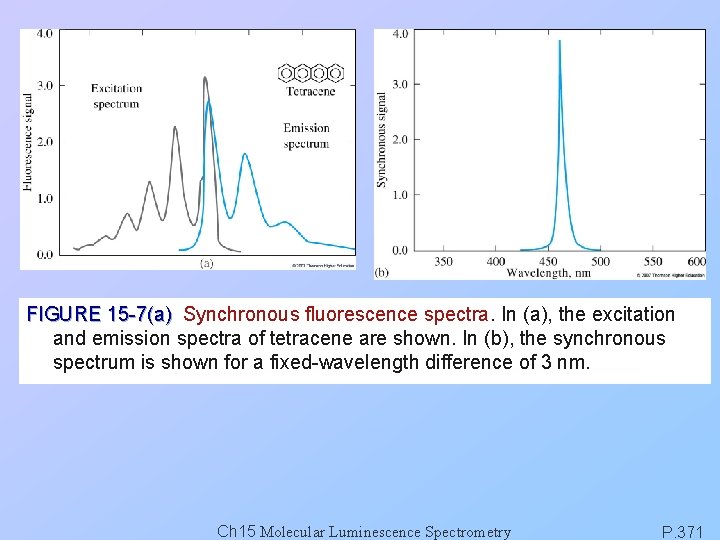
FIGURE 15 -7(a) Synchronous fluorescence spectra. In (a), the excitation and emission spectra of tetracene are shown. In (b), the synchronous spectrum is shown for a fixed-wavelength difference of 3 nm. Ch 15 Molecular Luminescence Spectrometry P. 371

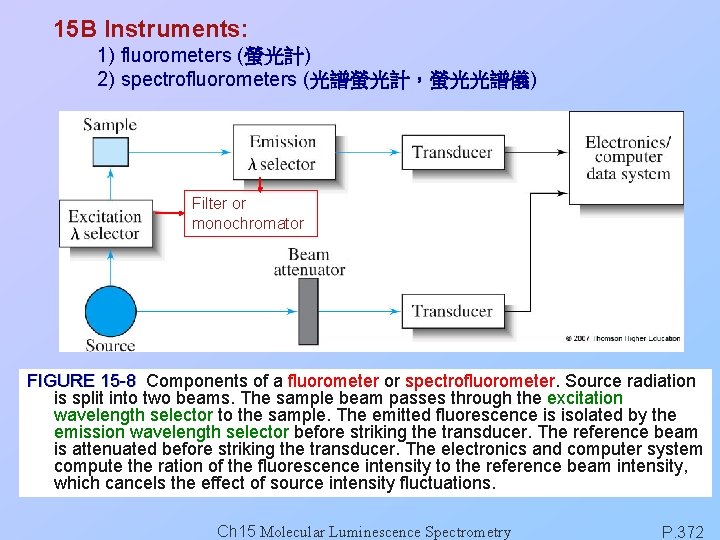
15 B Instruments: 1) fluorometers (螢光計) 2) spectrofluorometers (光譜螢光計,螢光光譜儀) Filter or monochromator FIGURE 15 -8 Components of a fluorometer or spectrofluorometer. Source radiation is split into two beams. The sample beam passes through the excitation wavelength selector to the sample. The emitted fluorescence is isolated by the emission wavelength selector before striking the transducer. The reference beam is attenuated before striking the transducer. The electronics and computer system compute the ration of the fluorescence intensity to the reference beam intensity, which cancels the effect of source intensity fluctuations. Ch 15 Molecular Luminescence Spectrometry P. 372

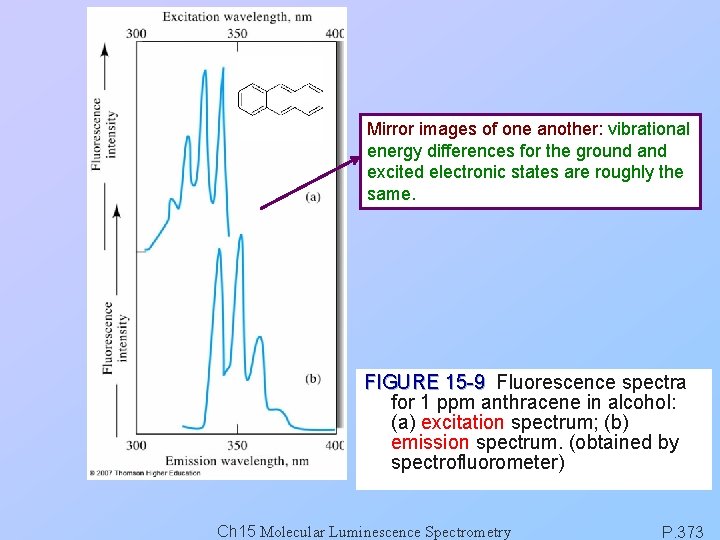
Mirror images of one another: vibrational energy differences for the ground and excited electronic states are roughly the same. FIGURE 15 -9 Fluorescence spectra for 1 ppm anthracene in alcohol: (a) excitation spectrum; (b) emission spectrum. (obtained by spectrofluorometer) Ch 15 Molecular Luminescence Spectrometry P. 373

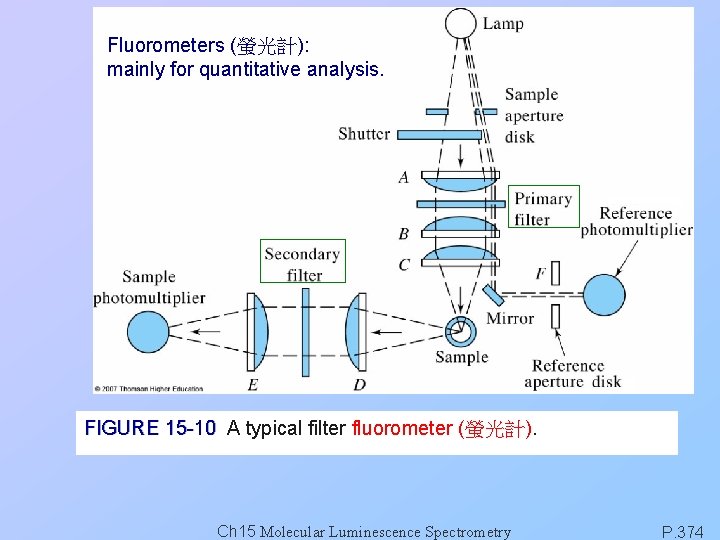
Fluorometers (螢光計): mainly for quantitative analysis. FIGURE 15 -10 A typical filter fluorometer (螢光計). Ch 15 Molecular Luminescence Spectrometry P. 374

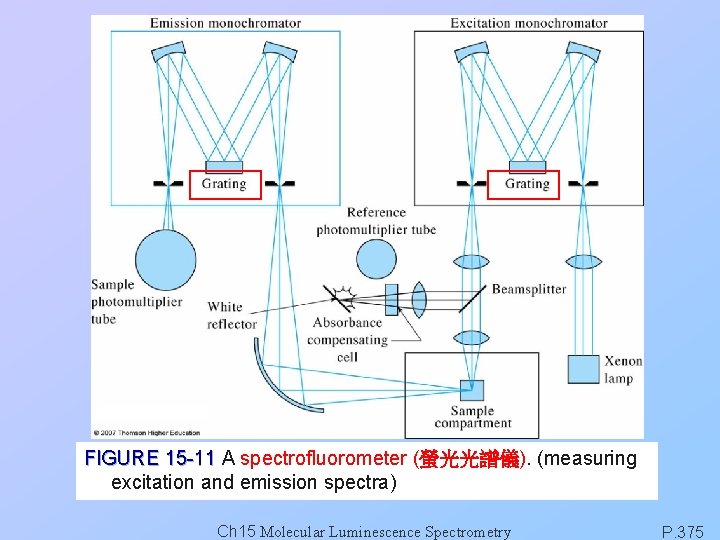
FIGURE 15 -11 A spectrofluorometer (螢光光譜儀). (measuring excitation and emission spectra) Ch 15 Molecular Luminescence Spectrometry P. 375

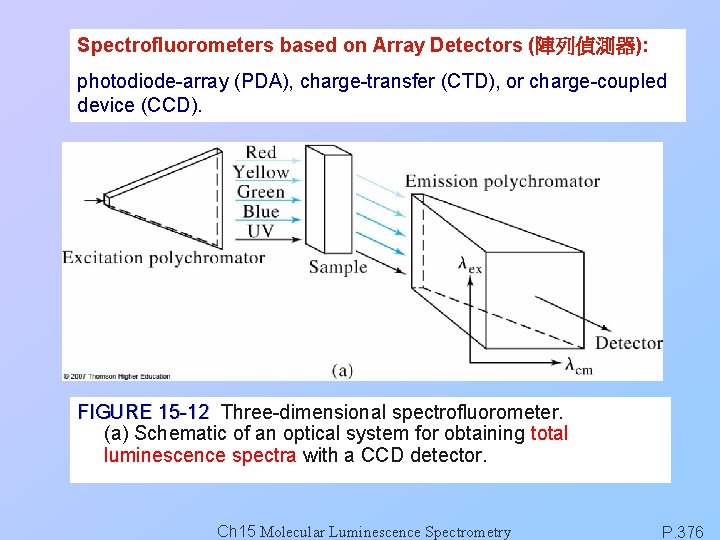
Spectrofluorometers based on Array Detectors (陣列偵測器): photodiode-array (PDA), charge-transfer (CTD), or charge-coupled device (CCD). FIGURE 15 -12 Three-dimensional spectrofluorometer. (a) Schematic of an optical system for obtaining total luminescence spectra with a CCD detector. Ch 15 Molecular Luminescence Spectrometry P. 376

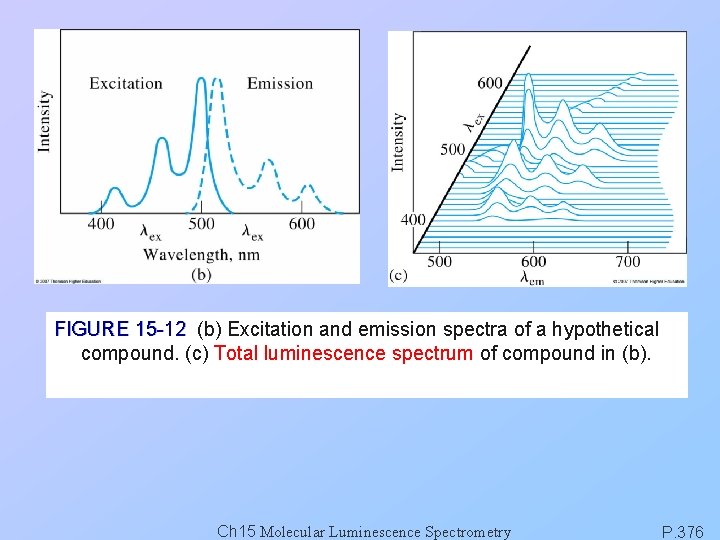
FIGURE 15 -12 (b) Excitation and emission spectra of a hypothetical compound. (c) Total luminescence spectrum of compound in (b). Ch 15 Molecular Luminescence Spectrometry P. 376

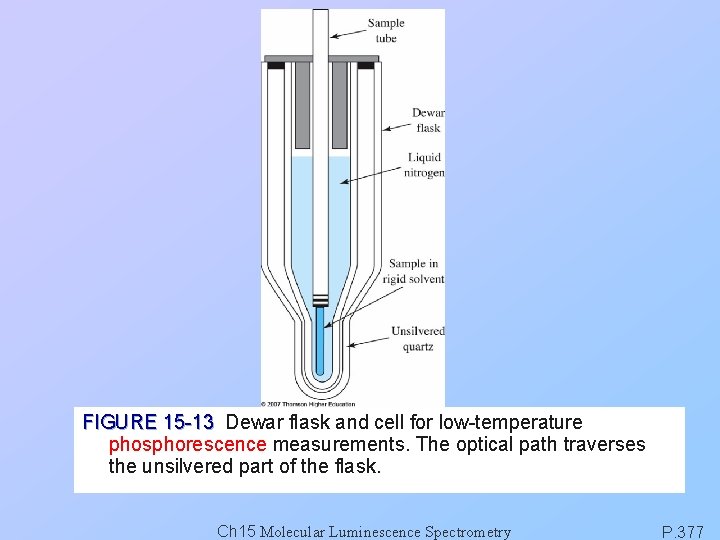
FIGURE 15 -13 Dewar flask and cell for low-temperature phosphorescence measurements. The optical path traverses the unsilvered part of the flask. Ch 15 Molecular Luminescence Spectrometry P. 377

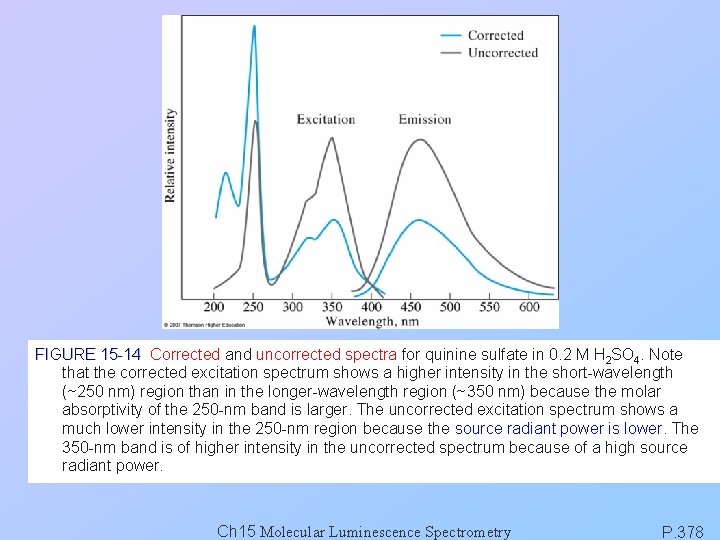
FIGURE 15 -14 Corrected and uncorrected spectra for quinine sulfate in 0. 2 M H 2 SO 4. Note that the corrected excitation spectrum shows a higher intensity in the short-wavelength (~250 nm) region than in the longer-wavelength region (~350 nm) because the molar absorptivity of the 250 -nm band is larger. The uncorrected excitation spectrum shows a much lower intensity in the 250 -nm region because the source radiant power is lower. The 350 -nm band is of higher intensity in the uncorrected spectrum because of a high source radiant power. Ch 15 Molecular Luminescence Spectrometry P. 378

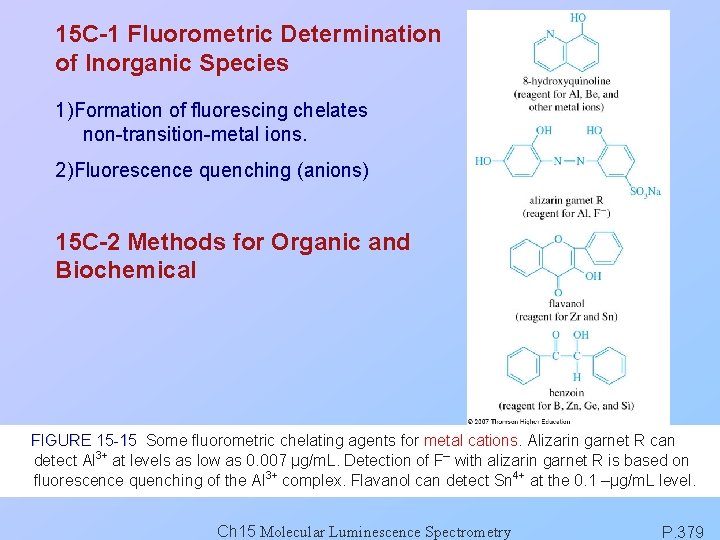
15 C-1 Fluorometric Determination of Inorganic Species 1)Formation of fluorescing chelates non-transition-metal ions. 2)Fluorescence quenching (anions) 15 C-2 Methods for Organic and Biochemical FIGURE 15 -15 Some fluorometric chelating agents for metal cations. Alizarin garnet R can detect Al 3+ at levels as low as 0. 007 μg/m. L. Detection of F– with alizarin garnet R is based on fluorescence quenching of the Al 3+ complex. Flavanol can detect Sn 4+ at the 0. 1 –μg/m. L level. Ch 15 Molecular Luminescence Spectrometry P. 379

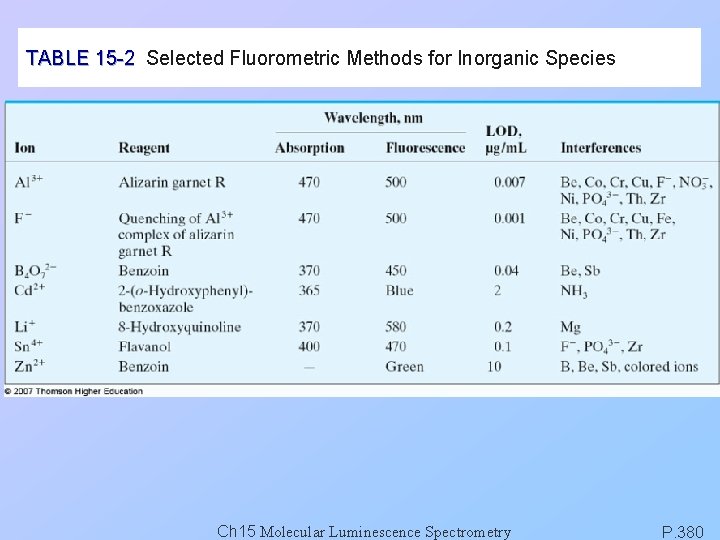
TABLE 15 -2 Selected Fluorometric Methods for Inorganic Species Ch 15 Molecular Luminescence Spectrometry P. 380

Review 1) 2) 3) Describe all possible relaxation processes of a singlet excited molecule. Why Stokes shift is constantly observed in molecular spectra? How about atomic spectra? Explain Stern-Volmer equation for fluorescence quenching.


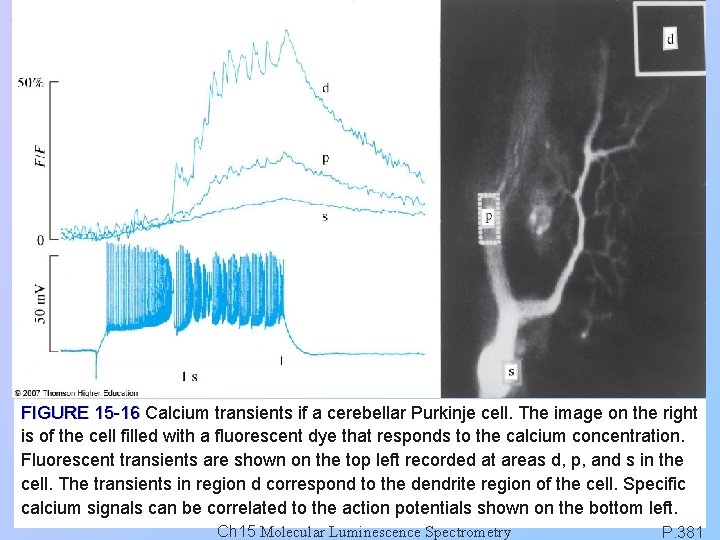
FIGURE 15 -16 Calcium transients if a cerebellar Purkinje cell. The image on the right is of the cell filled with a fluorescent dye that responds to the calcium concentration. Fluorescent transients are shown on the top left recorded at areas d, p, and s in the cell. The transients in region d correspond to the dendrite region of the cell. Specific calcium signals can be correlated to the action potentials shown on the bottom left. Ch 15 Molecular Luminescence Spectrometry P. 381

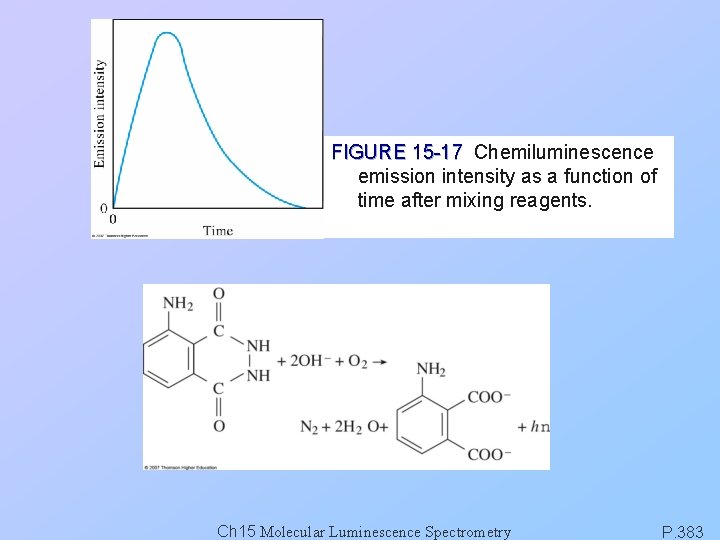
FIGURE 15 -17 Chemiluminescence emission intensity as a function of time after mixing reagents. Ch 15 Molecular Luminescence Spectrometry P. 383

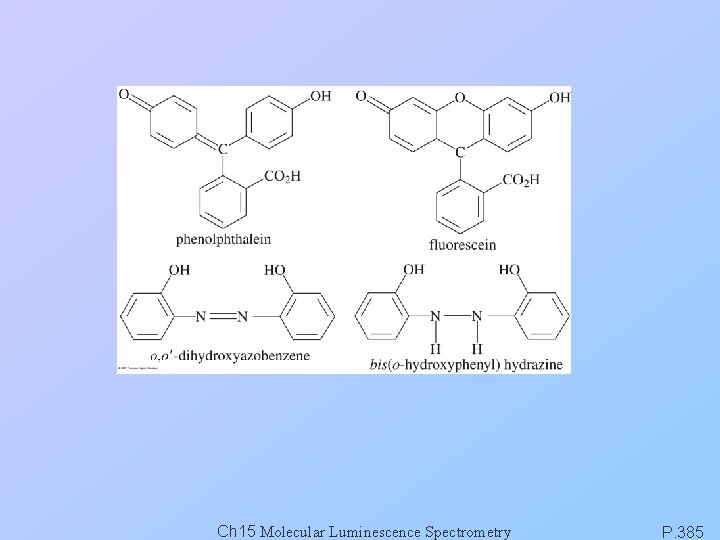
Ch 15 Molecular Luminescence Spectrometry P. 385