Kharkov National Medical University Department of Medical and





![A neutral solution, where [H+] = [OH-] An acidic solution, where [H+] [OH-] A A neutral solution, where [H+] = [OH-] An acidic solution, where [H+] [OH-] A](https://slidetodoc.com/presentation_image_h/97dcc18d98192f64f2373039c3e9fdc2/image-9.jpg)
![A way of expressing [OH–] is p. OH: p. OH = –lg[OH–] [H+][OH–] = A way of expressing [OH–] is p. OH: p. OH = –lg[OH–] [H+][OH–] =](https://slidetodoc.com/presentation_image_h/97dcc18d98192f64f2373039c3e9fdc2/image-11.jpg)



- Slides: 34

Kharkov National Medical University Department of Medical and Bioorganic Chemistry Medical Chemistry Lecture 3 ACID – BASE EQUILIBRIUM IN BIOLOGICAL SYSTEMS

SOLUTIONS Solutions are of great importance in the life and practical activities of man. A solution is a homogeneous mixture in which the components are uniformly intermingled. Solutions can be liquids or solids. All of the biological liquids – blood, lymph, inter cellular lymph, etc. – are solutions. All biochemical processes in organism occur in aqueous solutions.

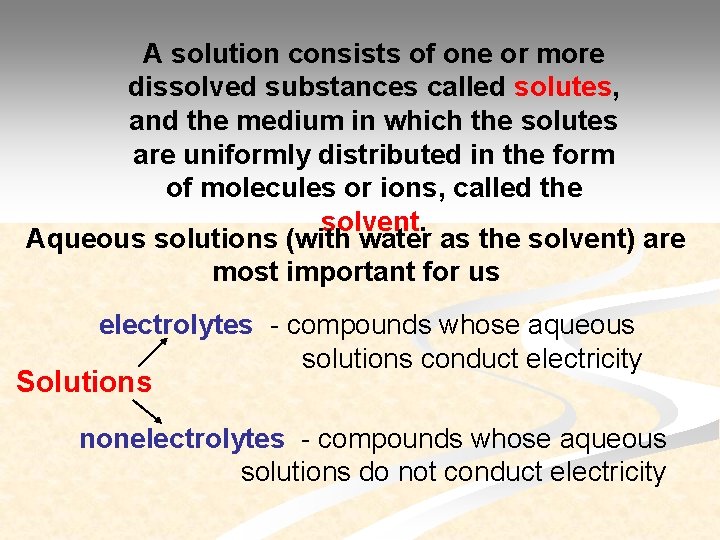
A solution consists of one or more dissolved substances called solutes, and the medium in which the solutes are uniformly distributed in the form of molecules or ions, called the solvent. Aqueous solutions (with water as the solvent) are most important for us electrolytes - compounds whose aqueous solutions conduct electricity Solutions nonelectrolytes - compounds whose aqueous solutions do not conduct electricity

There are two classes of electrolytes: strong electrolytes and weak electrolytes. Strong electrolytes dissociate virtually completely in aqueous solutions. Weak electrolytes dissociate only partly in aqueous solutions, and dynamic equilibrium sets in between the undissociated molecules and the ions in the solution.

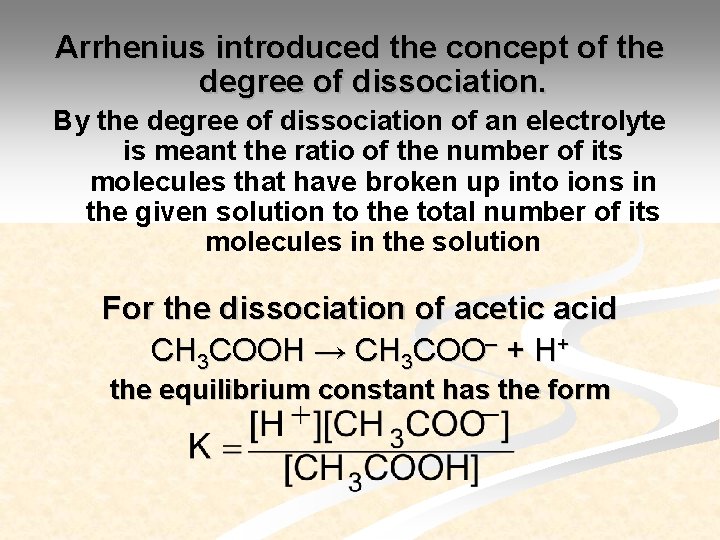
Arrhenius introduced the concept of the degree of dissociation. By the degree of dissociation of an electrolyte is meant the ratio of the number of its molecules that have broken up into ions in the given solution to the total number of its molecules in the solution For the dissociation of acetic acid CH 3 COOH → CH 3 COO– + H+ the equilibrium constant has the form

Cells of living organisms contain 60 -80% of water. Water is the most common amphoteric substance. Amphoteric substance - a substance which can behave either as an acid or as a base. H 2 O ↔ H + + OH- Water autoionization

The concentration of each ion at 25 C has been found by experiment to be 10 -7 mol/L. At 25 C [H+][OH-] = Kw (a constant) Kw =[10 -7] = 10 -14 Kw - the ion-product of water at 25 C or the dissociation constant

The meaning of Kw: In any aqueous solution at 25 C, no matter what it contains, the product of [H+] and [OH-] must always equal 10 -14
![A neutral solution where H OH An acidic solution where H OH A A neutral solution, where [H+] = [OH-] An acidic solution, where [H+] [OH-] A](https://slidetodoc.com/presentation_image_h/97dcc18d98192f64f2373039c3e9fdc2/image-9.jpg)
A neutral solution, where [H+] = [OH-] An acidic solution, where [H+] [OH-] A basic solution, where [OH-] [H+][OH ] = Kw=10 14

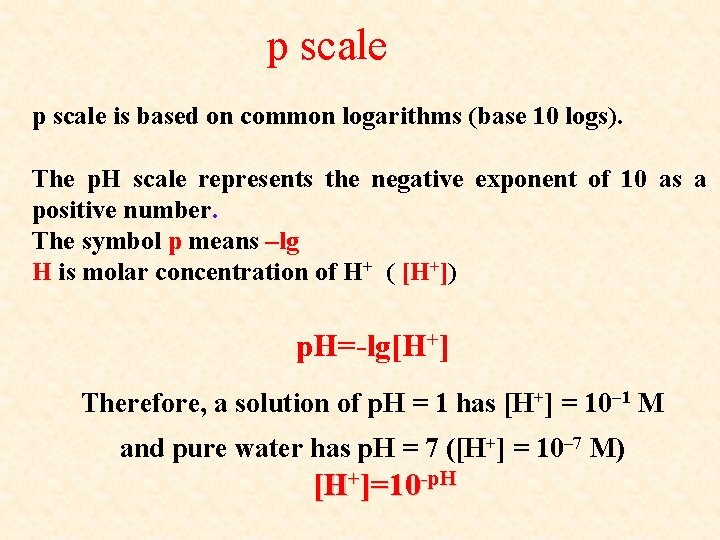
p scale is based on common logarithms (base 10 logs). The p. H scale represents the negative exponent of 10 as a positive number. The symbol p means –lg H is molar concentration of H+ ( [H+]) p. H=-lg[H+] Therefore, a solution of p. H = 1 has [H+] = 10– 1 M and pure water has p. H = 7 ([H+] = 10– 7 M) [H+]=10 -p. H
![A way of expressing OH is p OH p OH lgOH HOH A way of expressing [OH–] is p. OH: p. OH = –lg[OH–] [H+][OH–] =](https://slidetodoc.com/presentation_image_h/97dcc18d98192f64f2373039c3e9fdc2/image-11.jpg)
A way of expressing [OH–] is p. OH: p. OH = –lg[OH–] [H+][OH–] = 10– 14 If we now take lg of both sides of the equation, we hav –lg[H+][OH–] = –lg 10– 14 ( lg(A B) = lg. A + lg. B) lg[H+] lg[OH ] = lg 10 14

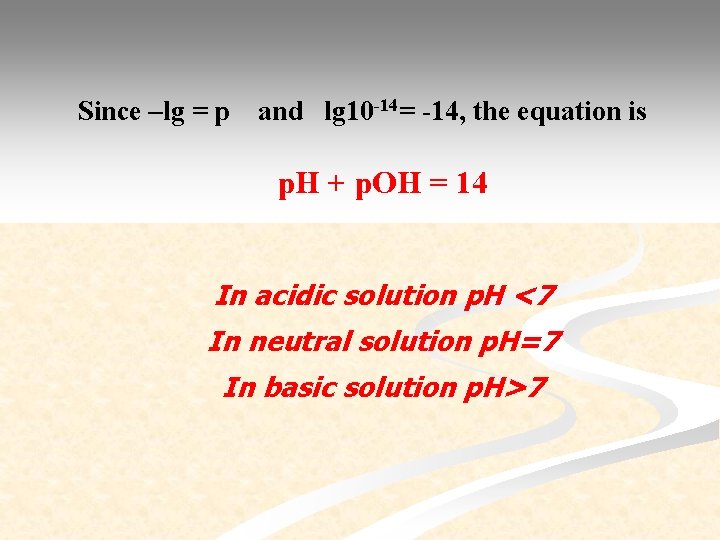
Since –lg = p and lg 10 -14= -14, the equation is p. H + p. OH = 14 In acidic solution p. H <7 In neutral solution p. H=7 In basic solution p. H>7

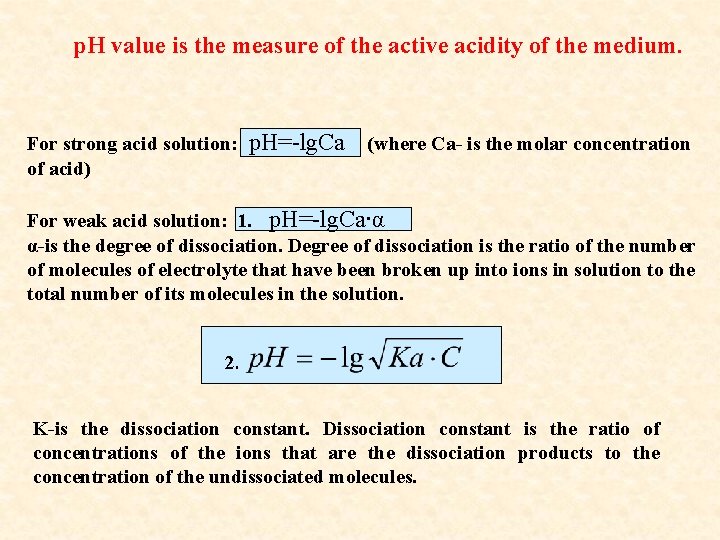
p. H value is the measure of the active acidity of the medium. For strong acid solution: of acid) p. H=-lg. Ca (where Ca- is the molar concentration For weak acid solution: 1. p. H=-lg. Ca∙α α-is the degree of dissociation. Degree of dissociation is the ratio of the number of molecules of electrolyte that have been broken up into ions in solution to the total number of its molecules in the solution. 2. K-is the dissociation constant. Dissociation constant is the ratio of concentrations of the ions that are the dissociation products to the concentration of the undissociated molecules.

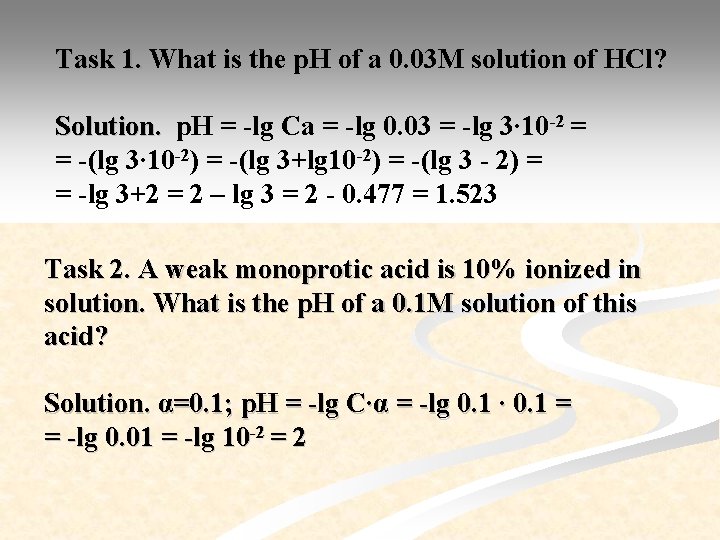
Task 1. What is the p. H of a 0. 03 M solution of HCl? Solution. p. H = -lg Ca = -lg 0. 03 = -lg 3∙ 10 -2 = = -(lg 3∙ 10 -2) = -(lg 3+lg 10 -2) = -(lg 3 - 2) = = -lg 3+2 = 2 – lg 3 = 2 - 0. 477 = 1. 523 Task 2. A weak monoprotic acid is 10% ionized in solution. What is the p. H of a 0. 1 M solution of this acid? Solution. α=0. 1; p. H = -lg C∙α = -lg 0. 1 ∙ 0. 1 = = -lg 0. 01 = -lg 10 -2 = 2

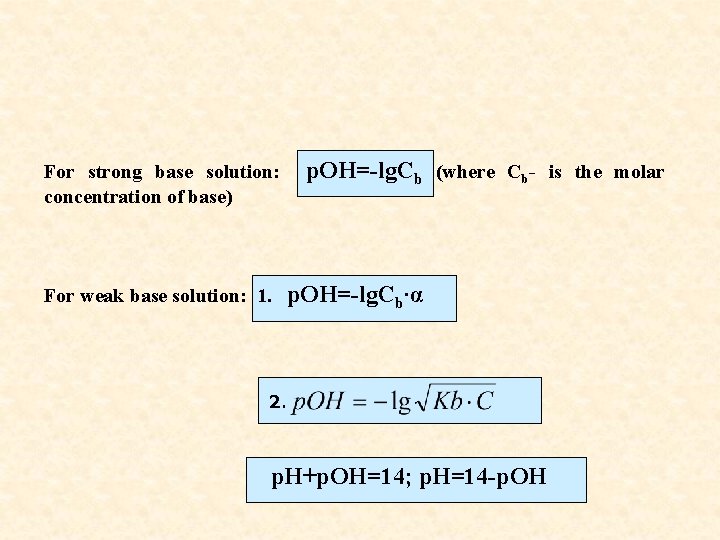
For strong base solution: concentration of base) For weak base solution: 1. p. OH=-lg. Cb (where Cb- is the molar p. OH=-lg. Cb∙α 2. p. H+p. OH=14; p. H=14 -p. OH

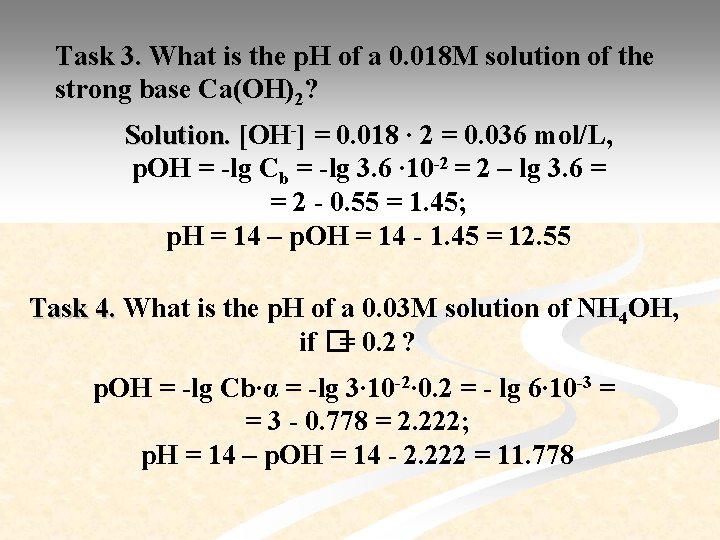
Task 3. What is the p. H of a 0. 018 M solution of the strong base Ca(OH)2? Solution. [OH-] = 0. 018 ∙ 2 = 0. 036 mol/L, p. OH = -lg Cb = -lg 3. 6 ∙ 10 -2 = 2 – lg 3. 6 = = 2 - 0. 55 = 1. 45; p. H = 14 – p. OH = 14 - 1. 45 = 12. 55 Task 4. What is the p. H of a 0. 03 M solution of NH 4 OH, if �= 0. 2 ? p. OH = -lg Cb∙α = -lg 3∙ 10 -2∙ 0. 2 = - lg 6∙ 10 -3 = = 3 - 0. 778 = 2. 222; p. H = 14 – p. OH = 14 - 2. 222 = 11. 778

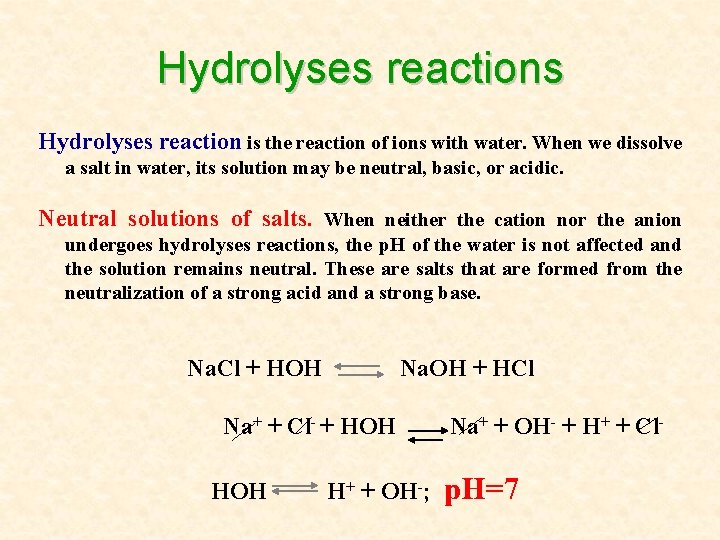
Hydrolyses reactions Hydrolyses reaction is the reaction of ions with water. When we dissolve a salt in water, its solution may be neutral, basic, or acidic. Neutral solutions of salts. When neither the cation nor the anion undergoes hydrolyses reactions, the p. H of the water is not affected and the solution remains neutral. These are salts that are formed from the neutralization of a strong acid and a strong base. Na. Cl + HOH Na. OH + HCl Na+ + Cl- + HOH H+ + OH-; Na+ + OH- + H+ + Cl- p. H=7

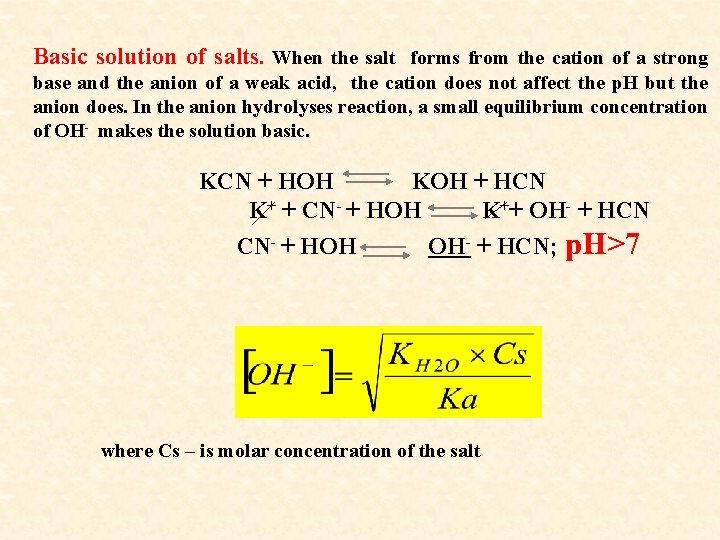
Basic solution of salts. When the salt forms from the cation of a strong base and the anion of a weak acid, the cation does not affect the p. H but the anion does. In the anion hydrolyses reaction, a small equilibrium concentration of OH- makes the solution basic. KCN + HOH KOH + HCN K+ + CN- + HOH K++ OH- + HCN CN- + HOH OH- + HCN; p. H>7 where Cs – is molar concentration of the salt

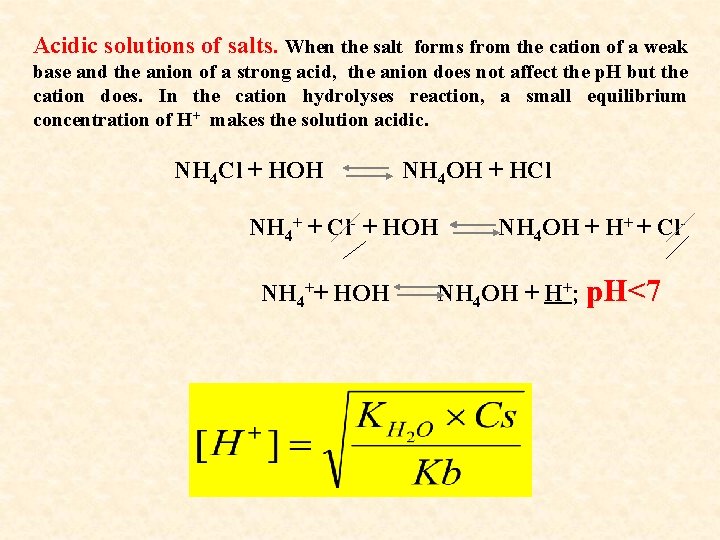
Acidic solutions of salts. When the salt forms from the cation of a weak base and the anion of a strong acid, the anion does not affect the p. H but the cation does. In the cation hydrolyses reaction, a small equilibrium concentration of H+ makes the solution acidic. NH 4 Cl + HOH NH 4 OH + HCl NH 4+ + Cl- + HOH NH 4++ HOH NH 4 OH + H+ + Cl- NH 4 OH + H+; p. H<7

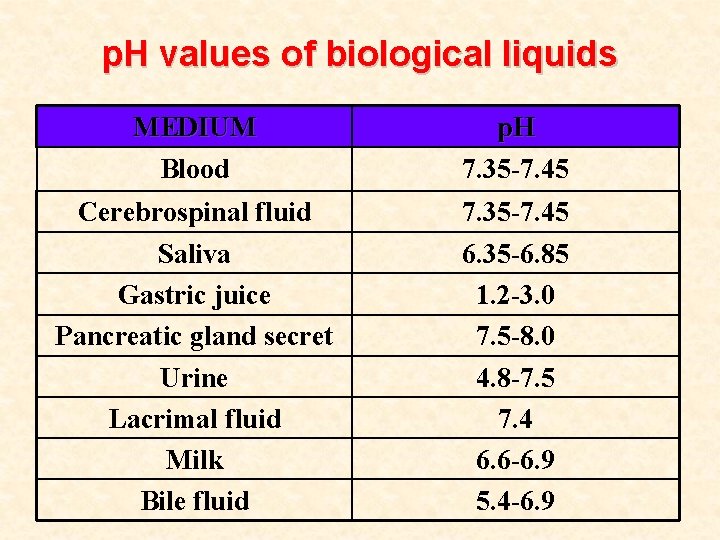
p. H values of biological liquids MEDIUM Blood p. H 7. 35 -7. 45 Cerebrospinal fluid Saliva Gastric juice Pancreatic gland secret Urine Lacrimal fluid Milk Bile fluid 7. 35 -7. 45 6. 35 -6. 85 1. 2 -3. 0 7. 5 -8. 0 4. 8 -7. 5 7. 4 6. 6 -6. 9 5. 4 -6. 9

A Buffer Solution (System) is one that resists a change in its p. H even when a strong acid or a base is added to it. BUFFER SOLUTION Acidic Basic Acidic Buffer: weak acid + its salt of a strong base HCN and Na. CN Basic Buffer: weak base + its salt of a strong acid NH 4 OH and NH 4 Cl

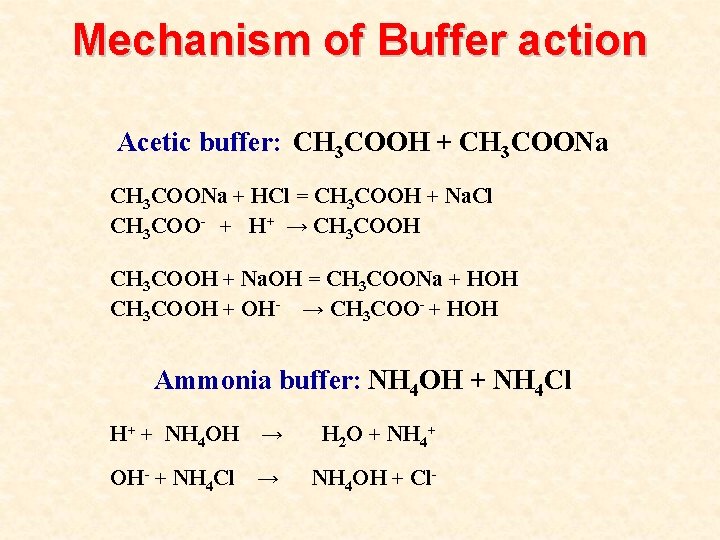
Mechanism of Buffer action Acetic buffer: CH 3 COOH + CH 3 COONa + HCl = CH 3 COOH + Na. Cl CH 3 COO- + H+ → CH 3 COOH + Na. OH = CH 3 COONa + HOH CH 3 COOH + OH- → CH 3 COO- + HOH Ammonia buffer: NH 4 OH + NH 4 Cl H+ + NH 4 OH → H 2 O + NH 4+ OH- + NH 4 Cl → NH 4 OH + Cl-

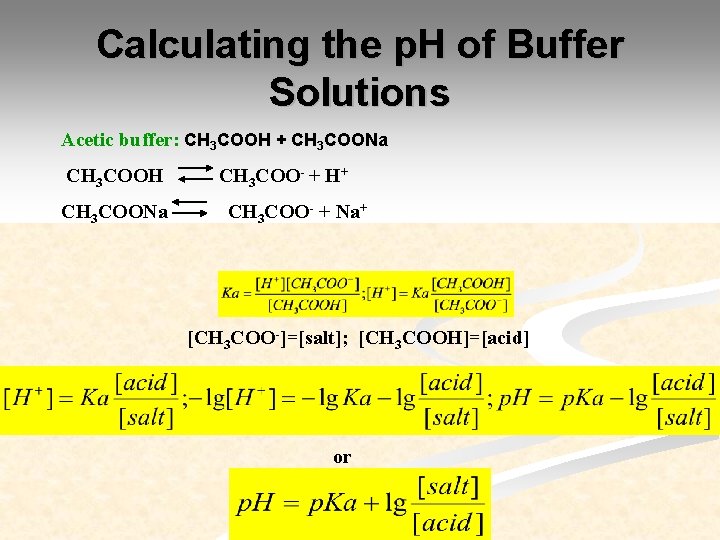
Calculating the p. H of Buffer Solutions Acetic buffer: CH 3 COOH + CH 3 COONa CH 3 COOH CH 3 COONa CH 3 COO- + H+ CH 3 COO- + Na+ [CH 3 COO-]=[salt]; [CH 3 COOH]=[acid] or

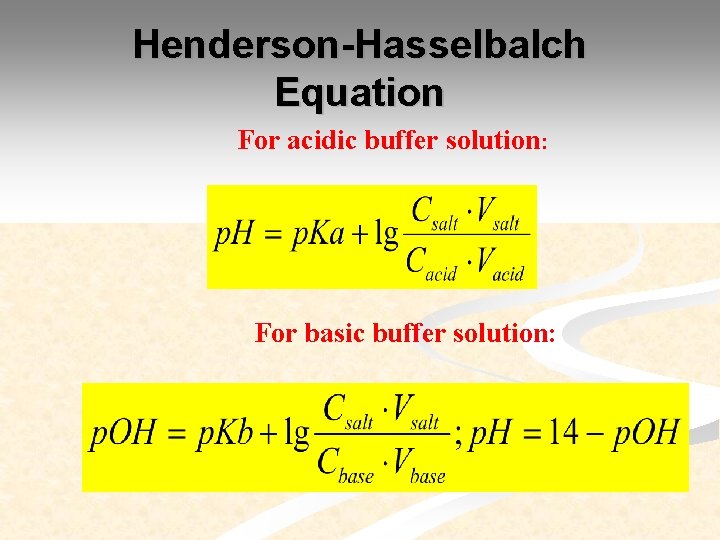
Henderson Hasselbalch Equation For acidic buffer solution: For basic buffer solution:

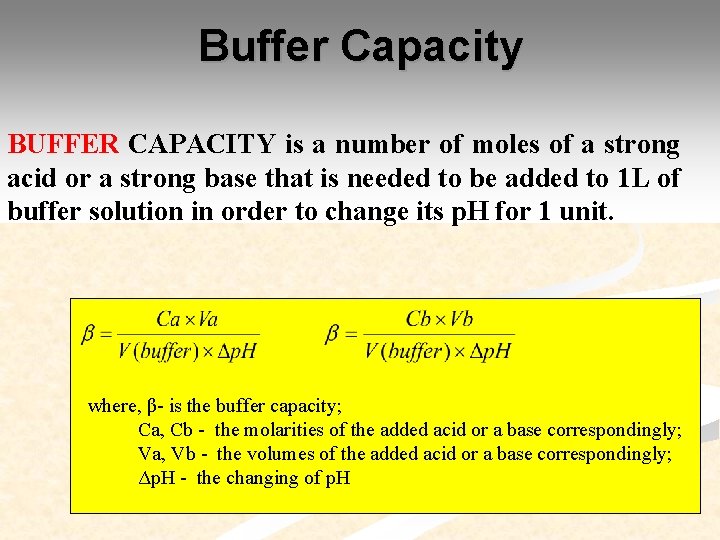
Buffer Capacity BUFFER CAPACITY is a number of moles of a strong acid or a strong base that is needed to be added to 1 L of buffer solution in order to change its p. H for 1 unit. where, β- is the buffer capacity; Ca, Cb - the molarities of the added acid or a base correspondingly; Va, Vb - the volumes of the added acid or a base correspondingly; Δp. H - the changing of p. H

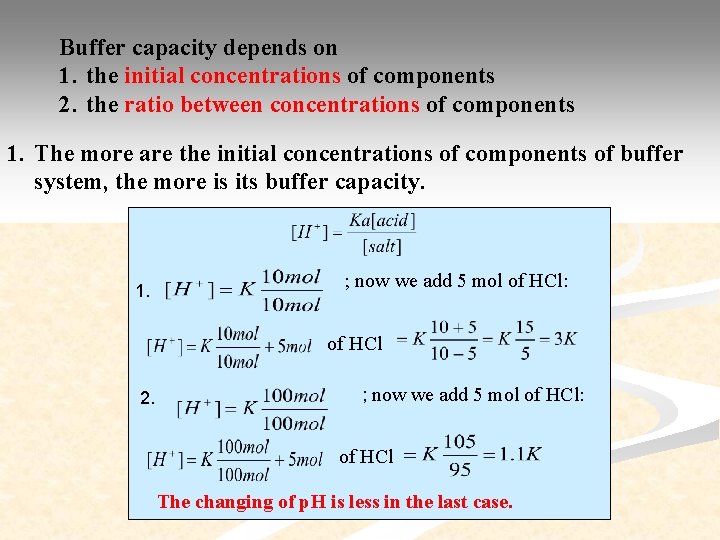
Buffer capacity depends on 1. the initial concentrations of components 2. the ratio between concentrations of components 1. The more are the initial concentrations of components of buffer system, the more is its buffer capacity. 1. ; now we add 5 mol of HCl: of HCl 2. ; now we add 5 mol of HCl: of HCl The changing of p. H is less in the last case.

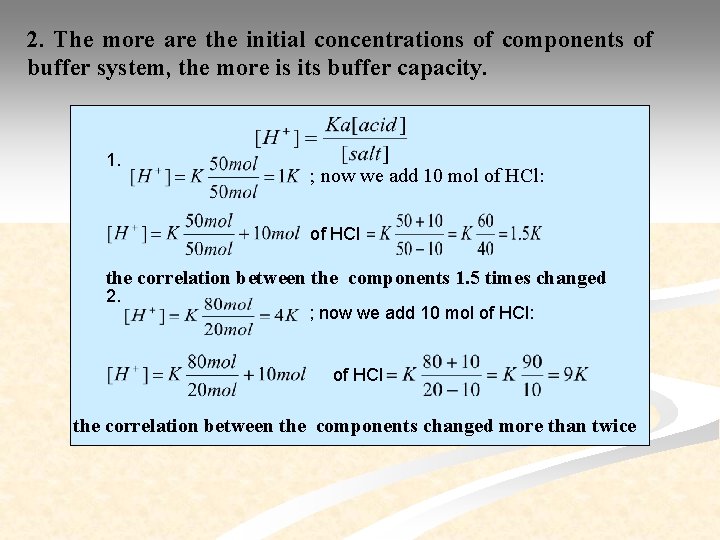
2. The more are the initial concentrations of components of buffer system, the more is its buffer capacity. 1. ; now we add 10 mol of HCl: of HCl the correlation between the components 1. 5 times changed 2. ; now we add 10 mol of HCl: of HCl the correlation between the components changed more than twice

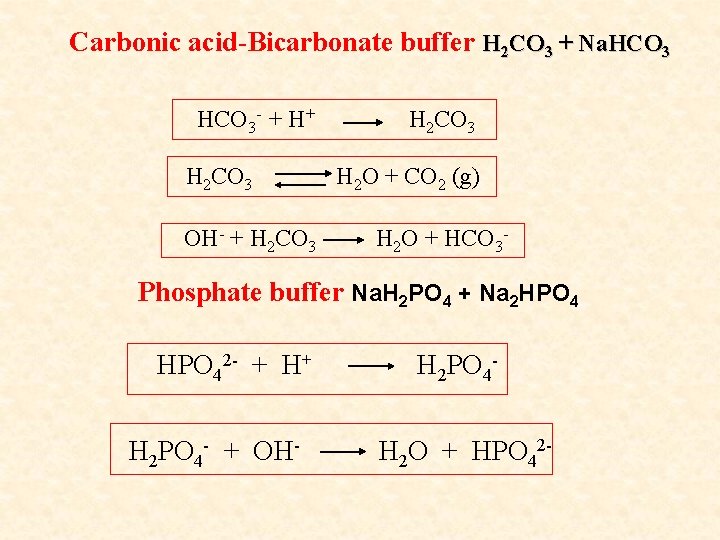
BUFFER SYSTEMS OF THE ORGANISM 1. Carbonic acid-Bicarbonate Buffer: H 2 CO 3 + Na. HCO 3 2. Phosphate Buffer: Na. H 2 PO 4 + Na 2 HPO 4 3. Amino Acid and Protein Buffer 4. Hemoglobinous Buffer: HHb + KHb 5. Oxyhemoglobinous Buffer: HHb. O 2 + KHb. O 2

Carbonic acid-Bicarbonate buffer H 2 CO 3 + Na. HCO 3 - + H+ H 2 CO 3 OH- + H 2 CO 3 H 2 O + CO 2 (g) H 2 O + HCO 3 - Phosphate buffer Na. H 2 PO 4 + Na 2 HPO 42 - + H+ H 2 PO 4 - + OH- H 2 PO 4 H 2 O + HPO 42 -

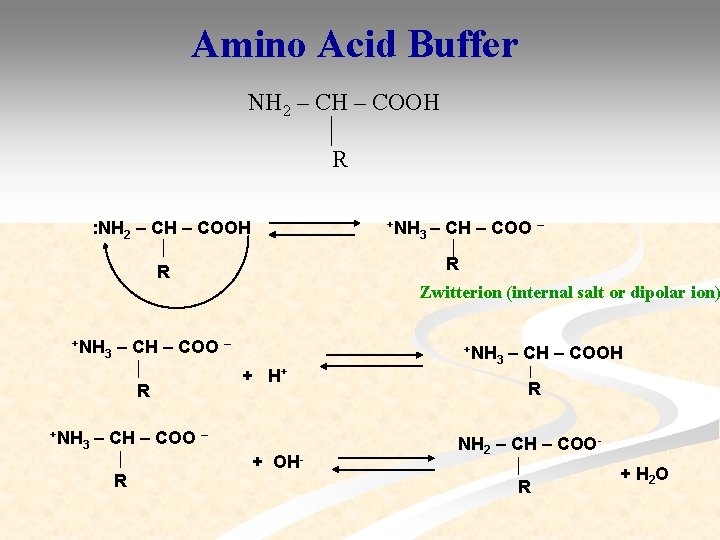
Amino Acid Buffer NH 2 – CH – COOH R : NH 2 – CH – COOH +NH 3– CH – COO – R R Zwitterion (internal salt or dipolar ion) +NH 3 – CH – COO – R +NH 3 +NH + H+ – CH – COO – R + OH 3 – CH – COOH R NH 2 – CH – COO R + H 2 O

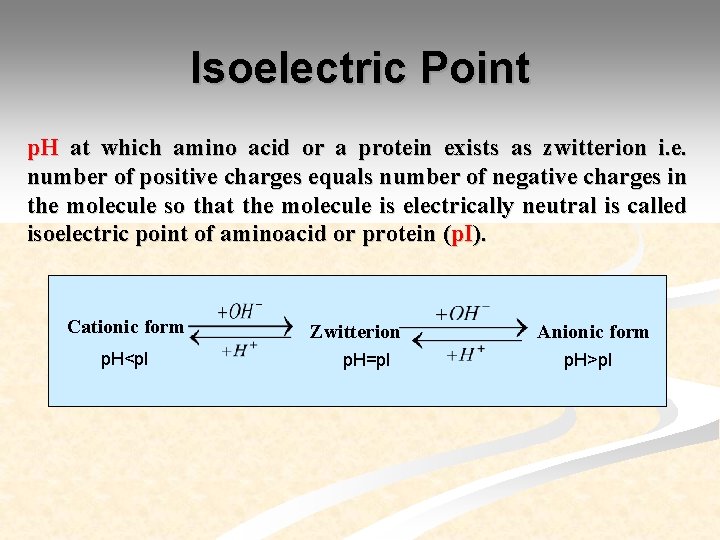
Isoelectric Point p. H at which amino acid or a protein exists as zwitterion i. e. number of positive charges equals number of negative charges in the molecule so that the molecule is electrically neutral is called isoelectric point of aminoacid or protein (p. I). Cationic form p. H<p. I Zwitterion p. H=p. I Anionic form p. H>p. I

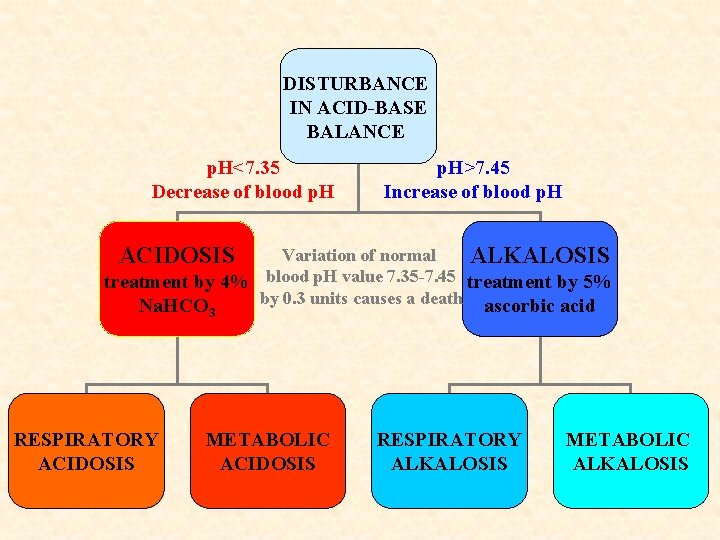
DISTURBANCE IN ACID-BASE BALANCE p. H<7. 35 Decrease of blood p. H>7. 45 Increase of blood p. H ACIDOSIS Variation of normal ALKALOSIS treatment by 4% blood p. H value 7. 35 -7. 45 treatment by 5% by 0. 3 units causes a death ascorbic acid Na. HCO 3 RESPIRATORY ACIDOSIS METABOLIC ACIDOSIS RESPIRATORY ALKALOSIS METABOLIC ALKALOSIS

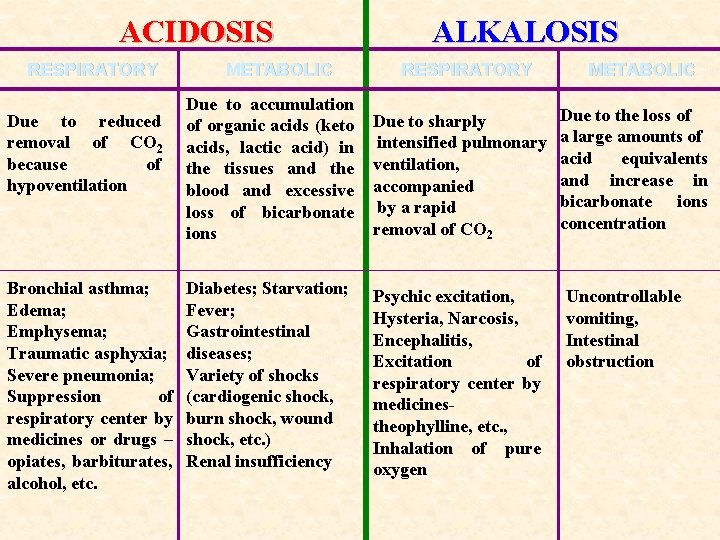
ACIDOSIS RESPIRATORY Due to reduced removal of CO 2 because of hypoventilation Bronchial asthma; Edema; Emphysema; Traumatic asphyxia; Severe pneumonia; Suppression of respiratory center by medicines or drugs – opiates, barbiturates, alcohol, etc. METABOLIC ALKALOSIS RESPIRATORY Due to accumulation of organic acids (keto acids, lactic acid) in the tissues and the blood and excessive loss of bicarbonate ions Due to sharply intensified pulmonary ventilation, accompanied by a rapid removal of CO 2 Diabetes; Starvation; Fever; Gastrointestinal diseases; Variety of shocks (cardiogenic shock, burn shock, wound shock, etc. ) Renal insufficiency Psychic excitation, Hysteria, Narcosis, Encephalitis, Excitation of respiratory center by medicinestheophylline, etc. , Inhalation of pure oxygen METABOLIC Due to the loss of a large amounts of acid equivalents and increase in bicarbonate ions concentration Uncontrollable vomiting, Intestinal obstruction

 M.gorky donetsk national medical university
M.gorky donetsk national medical university Seoul national university college of medicine
Seoul national university college of medicine Mongolian national university of medical sciences
Mongolian national university of medical sciences National risk and resilience unit
National risk and resilience unit Medical education and drugs department
Medical education and drugs department National core standards pdf
National core standards pdf National audit department
National audit department National unification and the national state
National unification and the national state Department of law university of jammu
Department of law university of jammu Department of geology university of dhaka
Department of geology university of dhaka University of padova psychology
University of padova psychology University of bridgeport it department
University of bridgeport it department University of iowa math department
University of iowa math department Sputonik
Sputonik Texas state majors
Texas state majors Department of information engineering university of padova
Department of information engineering university of padova Information engineering padova
Information engineering padova Manipal university chemistry department
Manipal university chemistry department Syracuse university psychology department
Syracuse university psychology department Jackson state university finance department
Jackson state university finance department Mice.cs.columbia
Mice.cs.columbia Michigan state university physics
Michigan state university physics Columbia university cs department
Columbia university cs department University of sargodha engineering department
University of sargodha engineering department Stanford university philosophy department
Stanford university philosophy department Kyiv national university of culture and arts
Kyiv national university of culture and arts National and kapodistrian university of athens events
National and kapodistrian university of athens events National yunlin university of science and technology
National yunlin university of science and technology Role of medical records
Role of medical records Ibn sina university of medical and pharmaceutical sciences
Ibn sina university of medical and pharmaceutical sciences Hepburn osteometric board
Hepburn osteometric board Chernivtsi national university
Chernivtsi national university National university of tainan
National university of tainan Lvivtech city
Lvivtech city Donetsk national technical university
Donetsk national technical university