IONIC COMPOUND FORMULATION NOMENCLATURE All ionic compounds consist


- Slides: 12

IONIC COMPOUND FORMULATION & NOMENCLATURE

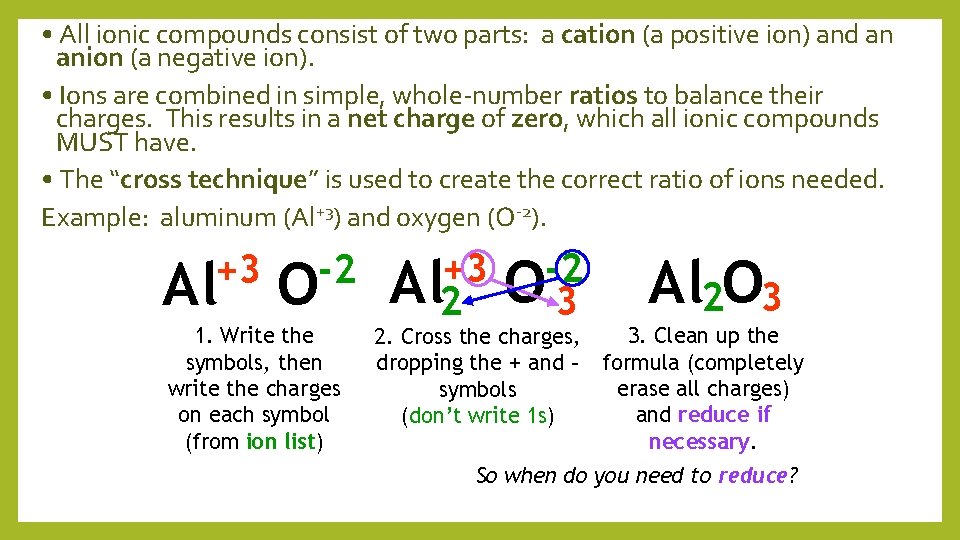
• All ionic compounds consist of two parts: a cation (a positive ion) and an anion (a negative ion). • Ions are combined in simple, whole-number ratios to balance their charges. This results in a net charge of zero, which all ionic compounds MUST have. • The “cross technique” is used to create the correct ratio of ions needed. Example: aluminum (Al+3) and oxygen (O-2). +3 -2 Al O 1. Write the symbols, then write the charges on each symbol (from ion list) +3 Al 2 -2 O 3 2. Cross the charges, dropping the + and – symbols (don’t write 1 s) Al 2 O 3 3. Clean up the formula (completely erase all charges) and reduce if necessary. So when do you need to reduce?

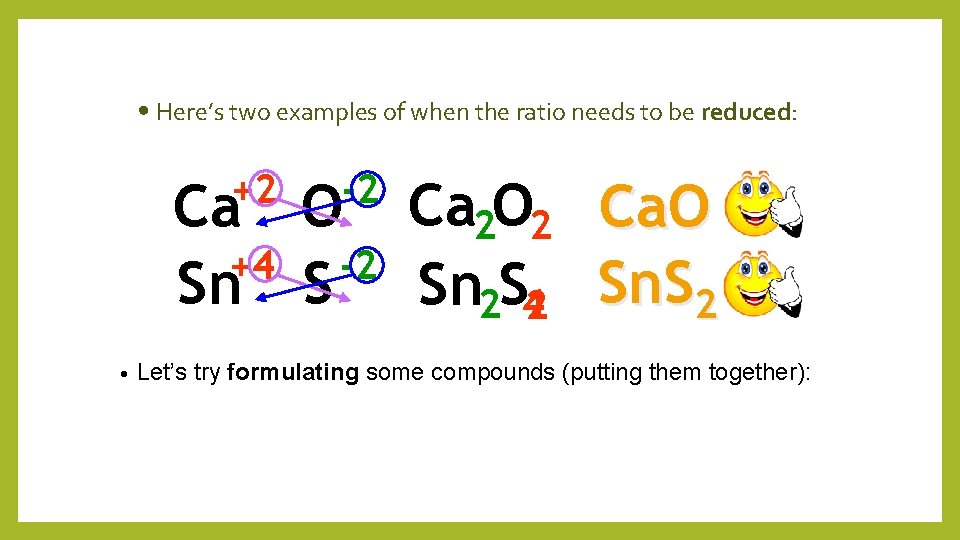
• Here’s two examples of when the ratio needs to be reduced: +2 -2 Ca O Ca 2 O 2 +4 -2 Sn S Sn 2 S 42 Ca. O Sn. S 2 • Let’s try formulating some compounds (putting them together):

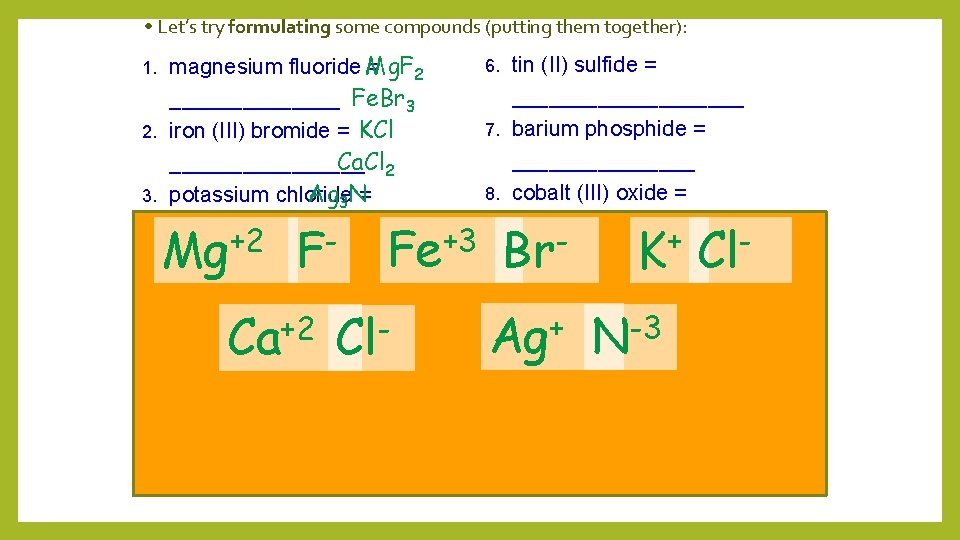
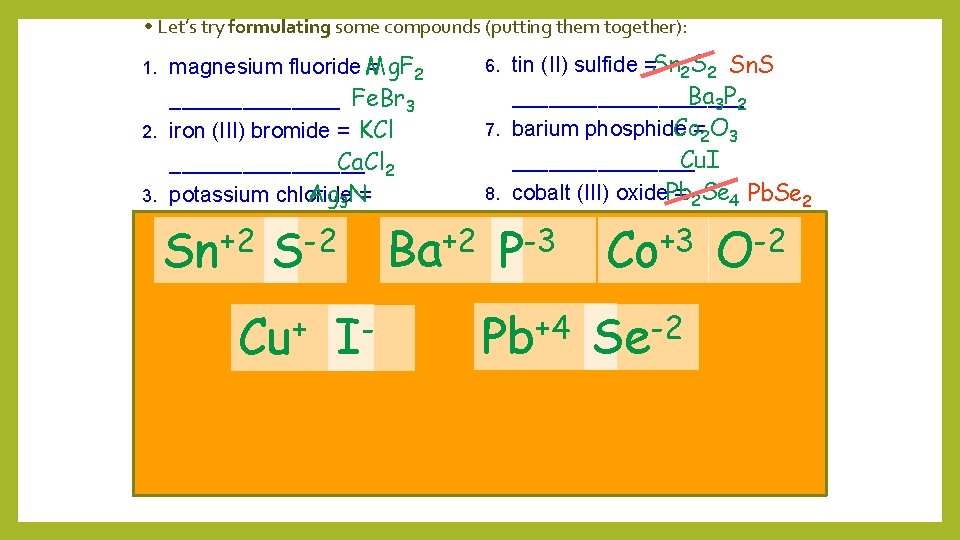
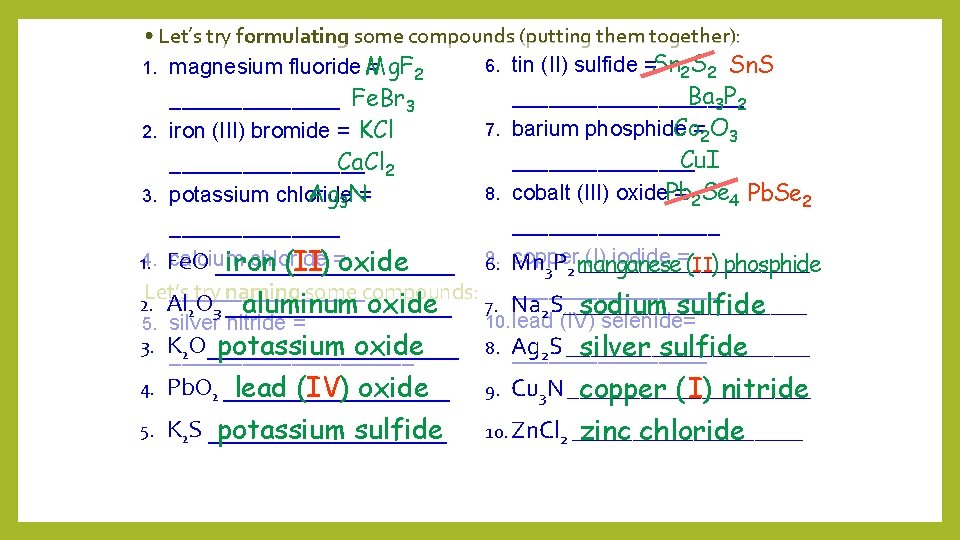
• Let’s try formulating some compounds (putting them together): magnesium fluoride Mg. F = 2 _______ Fe. Br 3 2. iron (III) bromide = KCl Ca. Cl 2 ________ Ag 3 N= 3. potassium chloride _______ +2 Let’s try naming some compounds: +3 calcium chloride = 1. 4. Fe. O __________ 2. Al 2 O 3 _________ 5. silver nitride = +2 3. K 2 O____________________ 6. 4. Pb. O 2 _________ 9. 5. K 2 S __________ 10. Zn. Cl 2 __________ 1. Mg Ca Fe F Cl tin (II) sulfide = __________ 7. barium phosphide = ________ 8. cobalt (III) oxide = _________ - (I) iodide+= 9. copper 6. Mn 3 P 2 __________ 7. Na 2 S__________ 10. lead (IV) selenide= + -3 8. Ag 2 S __________ Br K Cl Ag N Cu 3 N __________ MONATOMIC

• Let’s try formulating some compounds (putting them together): magnesium fluoride Mg. F = 2 _______ Fe. Br 3 2. iron (III) bromide = KCl Ca. Cl 2 ________ Ag 3 N= 3. potassium chloride _______ +2 -2 Let’s try naming some compounds: +2 calcium chloride = 1. 4. Fe. O __________ 2. Al 2 O 3 _________ 5. silver nitride = + 3. K 2 O____________________ 1. Sn S Ba Cu I tin (II) sulfide =Sn 2 S 2 Sn. S Ba 3 P 2 __________ Co=2 O 3 7. barium phosphide Cu. I ________ 8. cobalt (III) oxide. Pb = 2 Se 4 Pb. Se 2 _________ -3 (I) iodide+3 -2 9. copper = 6. Mn 3 P 2 __________ 7. Na 2 S__________ 10. lead (IV) selenide= +4 -2 8. Ag 2 S __________ 6. P Co Pb Se O 4. Pb. O 2 _________ 9. 5. K 2 S __________ 10. Zn. Cl 2 __________ Cu 3 N __________ MONATOMIC

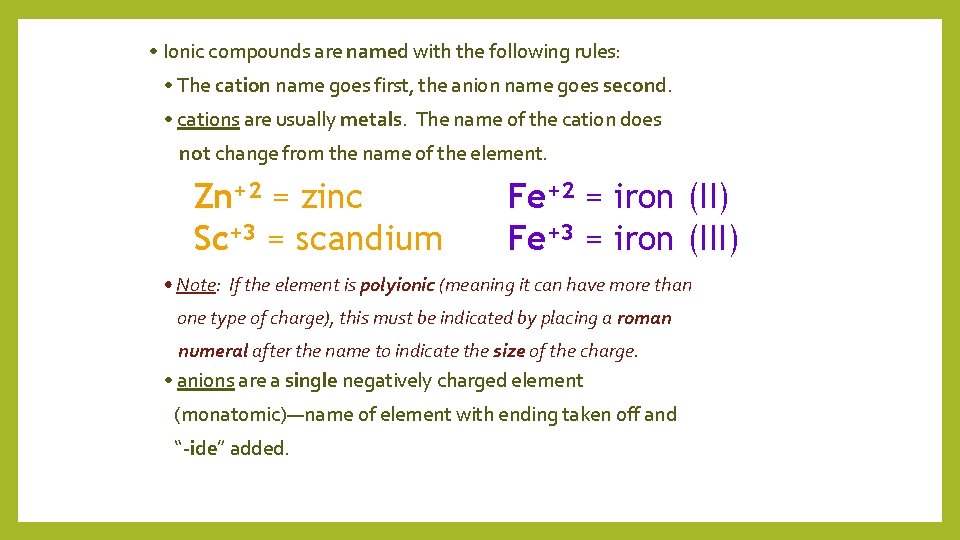
• Ionic compounds are named with the following rules: • The cation name goes first, the anion name goes second. • cations are usually metals. The name of the cation does not change from the name of the element. Zn+2 = zinc Sc+3 = scandium Fe+2 = iron (II) Fe+3 = iron (III) • Note: If the element is polyionic (meaning it can have more than one type of charge), this must be indicated by placing a roman numeral after the name to indicate the size of the charge. • anions are a single negatively charged element (monatomic)—name of element with ending taken off and “-ide” added.

• Let’s try formulating some compounds (putting them together): 6. tin (II) sulfide =Sn 2 S 2 Sn. S 1. magnesium fluoride Mg. F = 2 Ba 3 P 2 __________ Fe. Br 3 Co=2 O 3 7. barium phosphide 2. iron (III) bromide = KCl Cu. I ________ Ca. Cl 2 ________ 8. cobalt (III) oxide. Pb = 2 Se 4 Pb. Se 2 Ag 3 N= 3. potassium chloride _________ 9. copper (I) iodide =(II) phosphide calcium chloride 1. 4. Fe. O __________ 6. Mn __________ iron (II) =oxide 3 P 2 manganese ________ Let’s try naming some compounds: _________ 2. Al 2 O 3 _________ 7. Na 2 S__________ aluminum oxide sodium sulfide 10. lead (IV) selenide= 5. silver nitride = 3. K 2 O__________ 8. Ag potassium oxide silver sulfide 2 S ____________________ 4. Pb. O 2 _________ lead (IV) oxide 9. 5. K 2 S __________ potassium sulfide 10. Zn. Cl 2 __________ zinc chloride Cu 3 N __________ copper ( I) nitride

COVALENT BONDING CHARACTERISTICS AND NOMENCLATURE

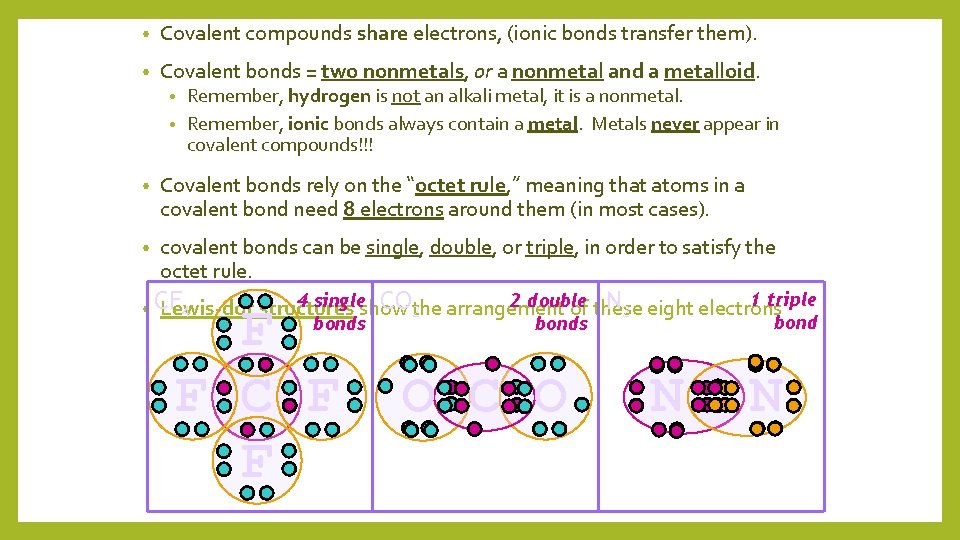
• Covalent compounds share electrons, (ionic bonds transfer them). • Covalent bonds = two nonmetals, or a nonmetal and a metalloid. Remember, hydrogen is not an alkali metal, it is a nonmetal. • Remember, ionic bonds always contain a metal. Metals never appear in covalent compounds!!! • • Covalent bonds rely on the “octet rule, ” meaning that atoms in a covalent bond need 8 electrons around them (in most cases). • covalent bonds can be single, double, or triple, in order to satisfy the octet rule. 1 triple 4 singleshow 2 double CO 2 the arrangement N 2 eight electrons • CF Lewis-dot structures of these 4 bonds F F C F F O C O N N

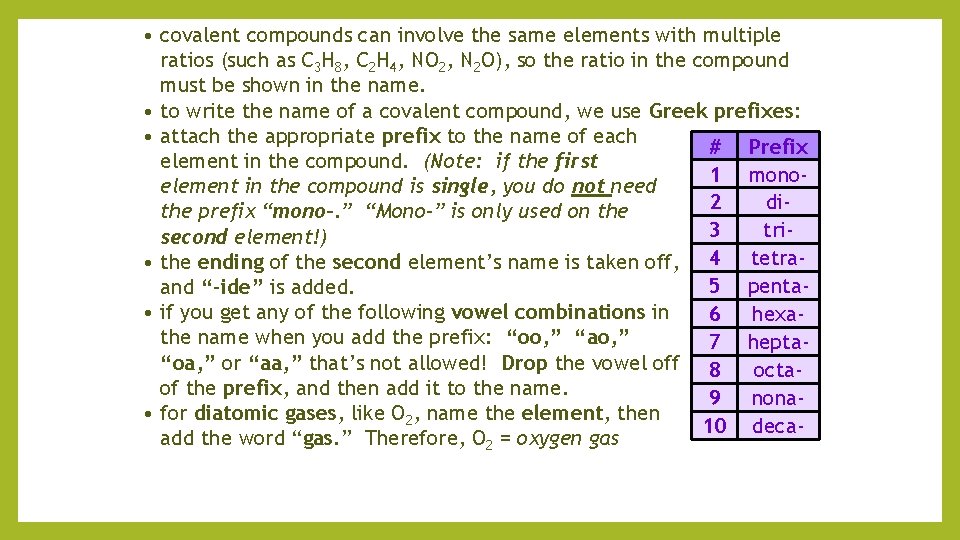
• covalent compounds can involve the same elements with multiple ratios (such as C 3 H 8, C 2 H 4, NO 2, N 2 O), so the ratio in the compound must be shown in the name. • to write the name of a covalent compound, we use Greek prefixes: • attach the appropriate prefix to the name of each # Prefix element in the compound. (Note: if the first 1 monoelement in the compound is single, you do not need 2 dithe prefix “mono-. ” “Mono-” is only used on the 3 trisecond element!) • the ending of the second element’s name is taken off, 4 tetra 5 pentaand “-ide” is added. • if you get any of the following vowel combinations in 6 hexathe name when you add the prefix: “oo, ” “ao, ” 7 hepta“oa, ” or “aa, ” that’s not allowed! Drop the vowel off 8 octaof the prefix, and then add it to the name. 9 nona • for diatomic gases, like O 2, name the element, then 10 decaadd the word “gas. ” Therefore, O 2 = oxygen gas

• Let’s try a few: 1. C 2 N 6 = ___________ dicarbon hexanitride 2. P 3 O 7 = ___________ triphosphorus heptaoxide heptoxide 6. no AO combo! no OO H 2 O = ___________ monooxide dihydrogen monoxide combo! no OA B 5 As = ___________ monoarsenide combo! pentaboron monarsenide N 2 = ____________ nitrogen gas (diatomic gas!) trisulfur tetrachloride = ______ S 3 Cl 4 7. carbon monoxide = ______ CO 8. Cl 2 (diatomic gas!) chlorine gas = ______ 3. 4. 5. 9. 10. Si. Br 5 silicon pentabromide = ______ Cl 4 Br 10 tetrachlorine decabromide = ______

Can you tell the difference between an ionic compound a covalent compound based on the name or formula alone? You need to be able to do so! Place an “I” or a “C” in the blank: I 1. Fe 3 N 2 ___ I 2. K 2 S ___ C 3. NH 3 ___ C 4. Br 2 ___ I 5. Mn 2 O 3 ___ I 6. Ca. O ___ I 7. Li 2 O ___ C 8. carbon dioxide ___ I 9. aluminum nitride ___ I 10. tin (II) sulfide ___ C 11. chlorine gas ___ I 12. calcium sulfide ___ I 13. cobalt (III) oxide ___ I 14. sodium phosphide ___