Introduction to Soil Engineering D A Cameron 2007




![Particle Interactions Coarse soils v. Fine soils [sand gravel] v. [silt and clay] STRENGTH Particle Interactions Coarse soils v. Fine soils [sand gravel] v. [silt and clay] STRENGTH](https://slidetodoc.com/presentation_image_h/9af25574ace8445bb2cb6acf80a7456c/image-8.jpg)














- Slides: 38

Introduction to Soil Engineering D. A. Cameron 2007 DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 1

Staff CIVIL ENGINEERING Dr. Don Cameron donald. cameron@unisa. edu. au P 2 -35 ph 8302 3128 DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 2

Reference Barnes, G E “Soil Mechanics, Principles and Practice, ” Mac. Millan Press Civil Engineering students will need this text in 3 rd year DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 3

The engineering behaviour of soil 1. 2. 3. 4. 5. 6. 7. 8. 9. How soils are formed The basic units which form soil material Engineering concepts of sand, silt and clay The Unified Soil Classification System Stress in soil, total and effective Water flow in saturated soils Erosion, scour or piping Physical improvement of soil (“compaction”) Terminology DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 4

Origins of Soils • Residual • Glacial • Alluvial • Marine • Aeolian = wind blown • Lacustrine • Organic DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 5

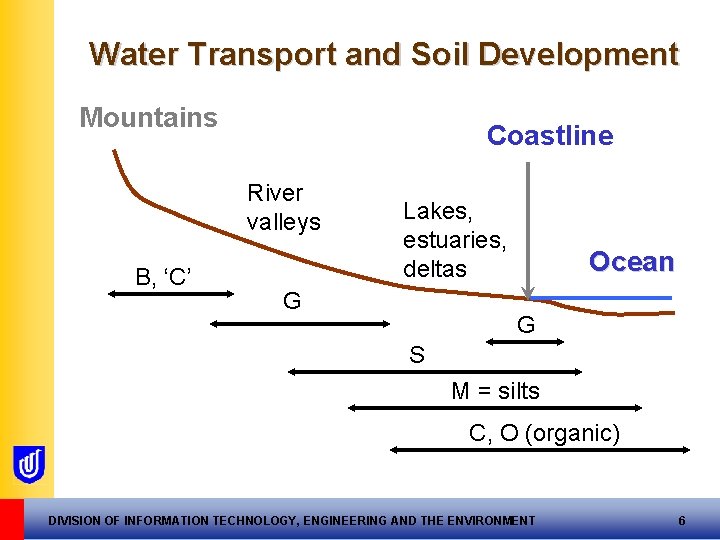
Water Transport and Soil Development Mountains Coastline River valleys B, ‘C’ Lakes, estuaries, deltas G Ocean G S M = silts C, O (organic) DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 6

Soil from Rocks = Residual SAND - quartz, silica SILT - finer quartz & silica (8: 4: 2) CLAY - clay minerals (from weathered feldspar & mica ) − very fine “clay” particles DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 7
![Particle Interactions Coarse soils v Fine soils sand gravel v silt and clay STRENGTH Particle Interactions Coarse soils v. Fine soils [sand gravel] v. [silt and clay] STRENGTH](https://slidetodoc.com/presentation_image_h/9af25574ace8445bb2cb6acf80a7456c/image-8.jpg)
Particle Interactions Coarse soils v. Fine soils [sand gravel] v. [silt and clay] STRENGTH DERIVED FROM Friction, interlock v. physico-chemical interaction DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 8

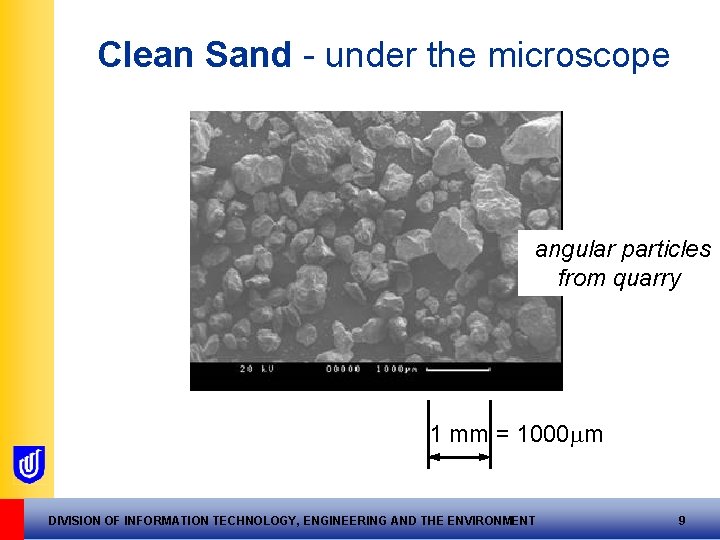
Clean Sand - under the microscope angular particles from quarry 1 mm = 1000 m DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 9

Fine - Grained Soils Cohesion “Apparent” cohesion “apparent” tensile strength, arising from electrostatic forces (are stronger, the finer the particle) DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 10

Molecular Structure of the Clay Minerals Lecture 1 Civil Engineering Practice DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 11

http: //pubpages. unh. edu/~harter/crystal. htm# Phyllosilicates are the clay “building blocks” Tetrahedrons & Octahedrons • Clays form from weathering and secondary sedimentary processes • Clays are usually mixed − other clays − microscopic crystals of carbonates, feldspars, micas and quartz DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 12

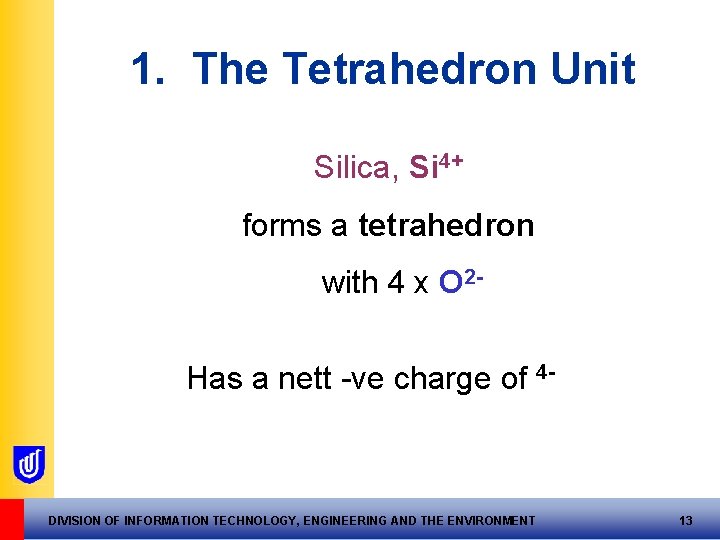
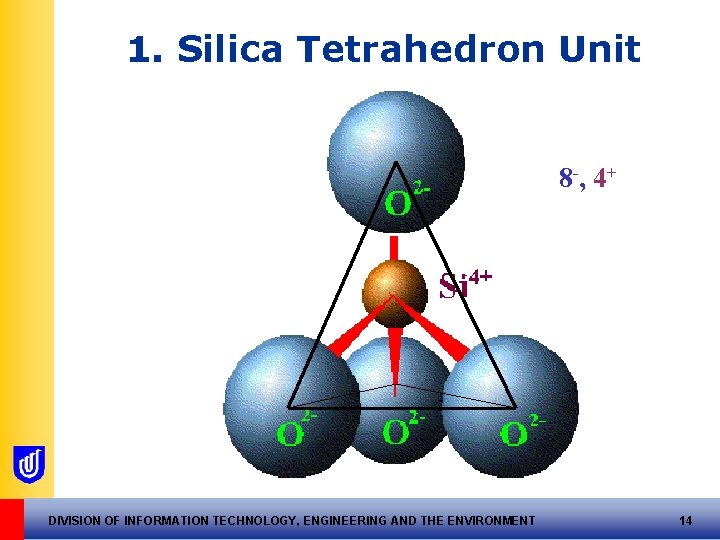
1. The Tetrahedron Unit Silica, Si 4+ forms a tetrahedron with 4 x O 2 Has a nett -ve charge of 4 - DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 13

1. Silica Tetrahedron Unit 8 -, 4+ DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 14

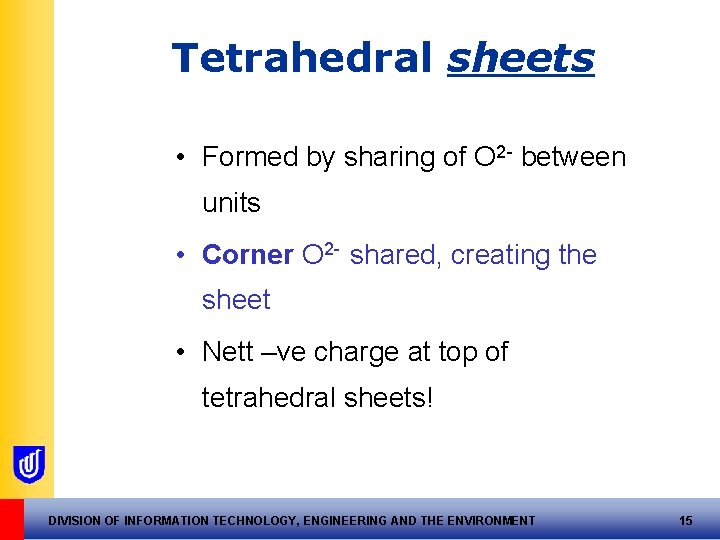
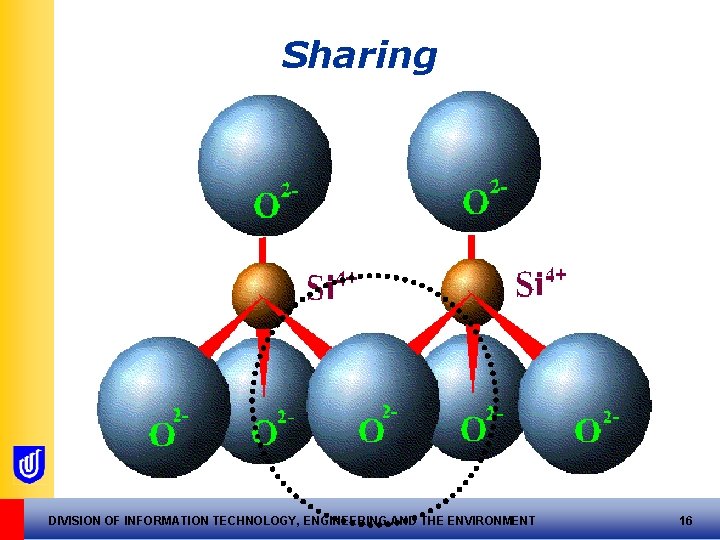
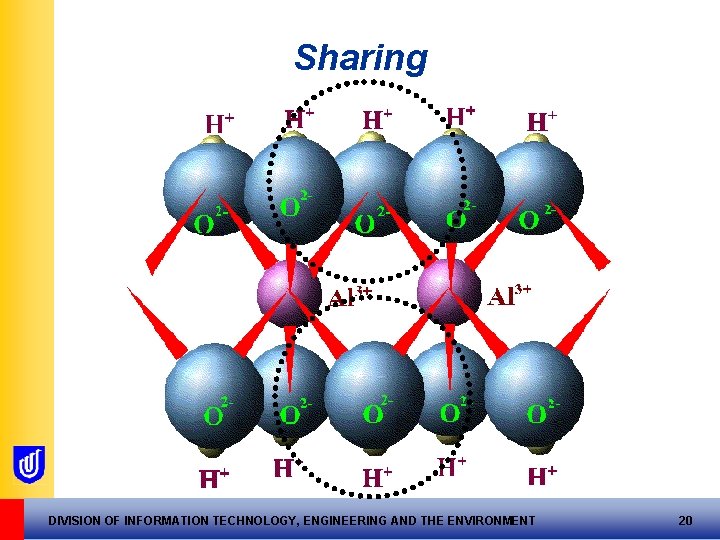
Tetrahedral sheets • Formed by sharing of O 2 - between units • Corner O 2 - shared, creating the sheet • Nett –ve charge at top of tetrahedral sheets! DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 15

Sharing DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 16

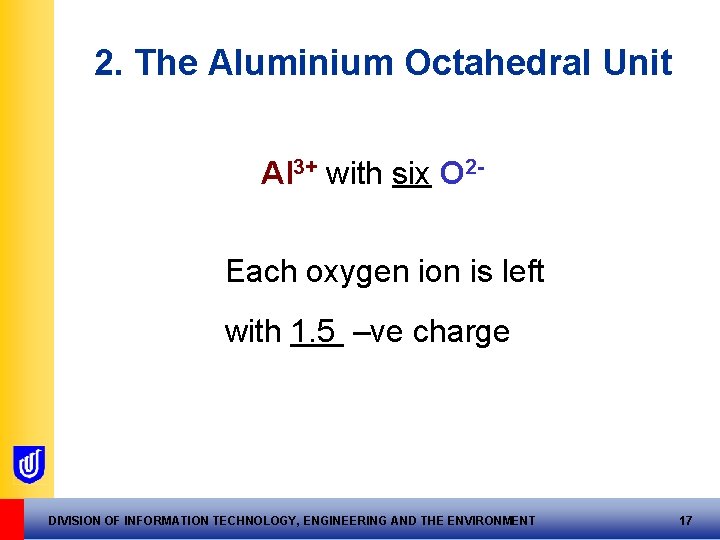
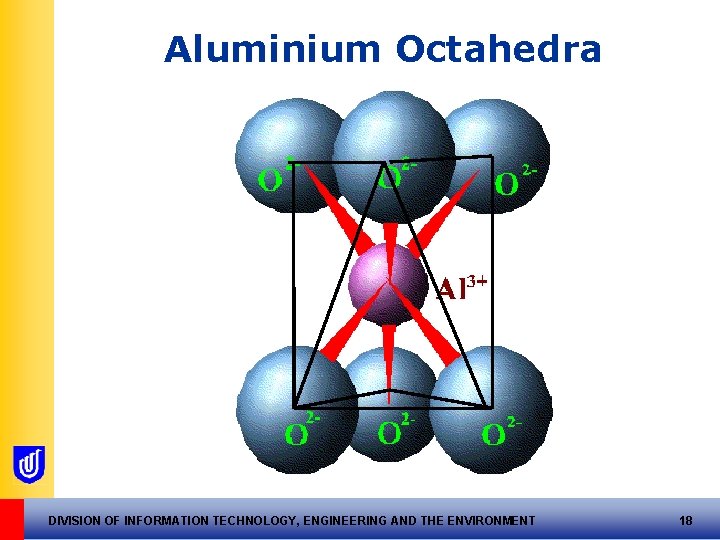
2. The Aluminium Octahedral Unit Al 3+ with six O 2 Each oxygen ion is left with 1. 5 –ve charge DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 17

Aluminium Octahedra DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 18

Octahedral sheets • Octahedral sheets formed by each oxygen being bonded to two Al ions • Each O ion left with one –ve charge • IF charge satisfied by hydrogen ions, the Gibbsite mineral is formed DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 19

Sharing DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 20

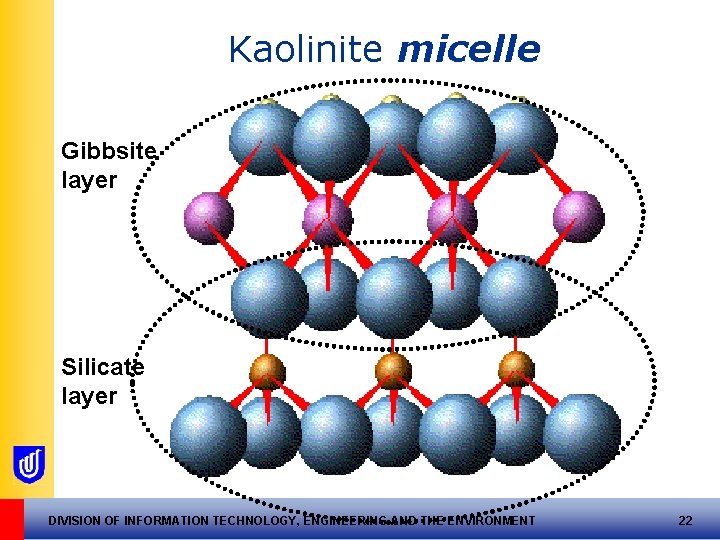
The Kaolinite CLAY Mineral • Top oxygen ions in Silica sheet bonded to Aluminium sheet – “ 1: 1 clay mineral” • Each top oxygen ion shared by 2 Al and 1 O ion • This unit = “a clay micelle” (approx. 0. 7 nm thick and 10 x 10 nm) DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 21

Kaolinite micelle Gibbsite layer Silicate layer DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 22

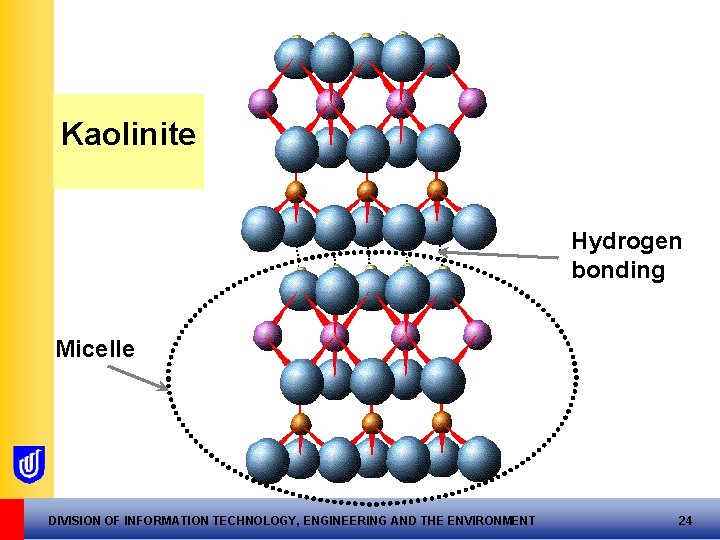
Kaolinite clay mineral …consists of stacks of micelles Usually hydrogen bonds micelles together: a strong bond stable clay mineral DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 23

Kaolinite Hydrogen bonding Micelle DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 24

Kaolinite DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 25

2: 1 Clay Minerals “The Mica Group” 3 sheets, 2 silica tetrahedra, 1 aluminium octahedron = a micelle Many different clay minerals occur with this basic unit e. g. “Illite” (Adelaide clays) and “Montmorillonite” (basaltic clays) DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 26

Smectite (includes montmorillonite) DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 27

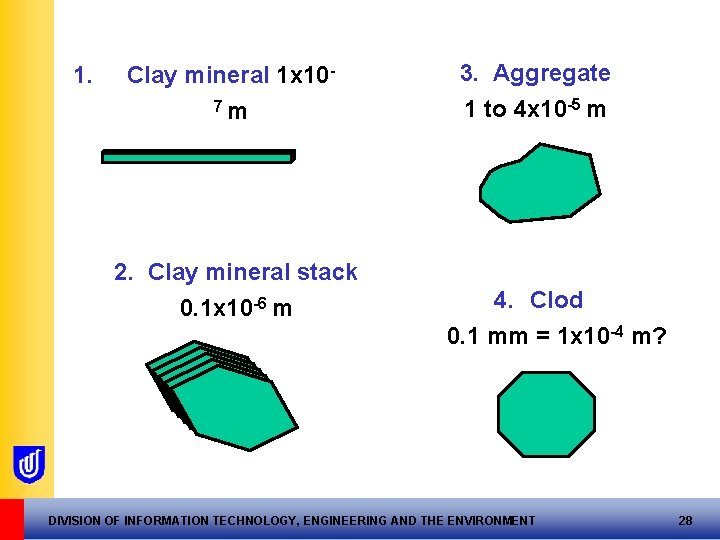
1. Clay mineral 1 x 107 m 2. Clay mineral stack 0. 1 x 10 -6 m 3. Aggregate 1 to 4 x 10 -5 m 4. Clod 0. 1 mm = 1 x 10 -4 m? DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 28

Properties of the clay minerals When mixed with a little water, clays become “plastic” i. e. are able to be moulded SO, moisture affects clay soil engineering properties DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 29

Properties of the clay minerals • Can absorb or lose water between the silicate sheets − • negative charge attracts H 2 O When water is absorbed, clays may Expand ! − water in spaces between stacked layers − Montmorillonite most expandable − Kaolinite the least DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 30

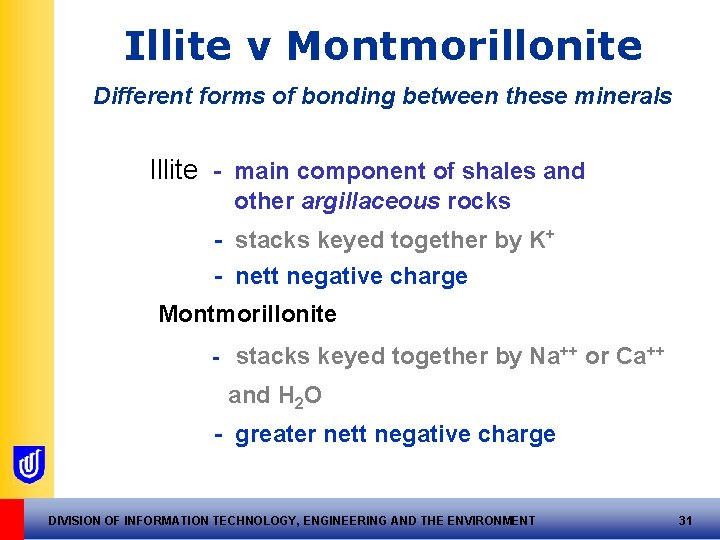
Illite v Montmorillonite Different forms of bonding between these minerals Illite - main component of shales and other argillaceous rocks - stacks keyed together by K+ - nett negative charge Montmorillonite - stacks keyed together by Na++ or Ca++ and H 2 O - greater nett negative charge DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 31

Clay Minerals – capacity for water i) Kaolinite (China clay) Water absorption, approximately 90% ii) Montmorillonite (Bentonite, Smectite) Water absorption, approximately 300 - 700% iii) Illite Intermediate water absorption DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 32

“Specific surface” = grain area/grain mass DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 33

The influence of charges “The greater the surface area, the greater the charge” − the greater the affinity for water − some water strongly adsorbed in a very thin layer − other water “free” in the soil “pores” Electrostatic forces give rise to COHESION in soils with clay minerals DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 34

Uses of Kaolinite q Ceramics (China clay) q Filler for paint, rubber & plastics q Glossy paper production DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 35

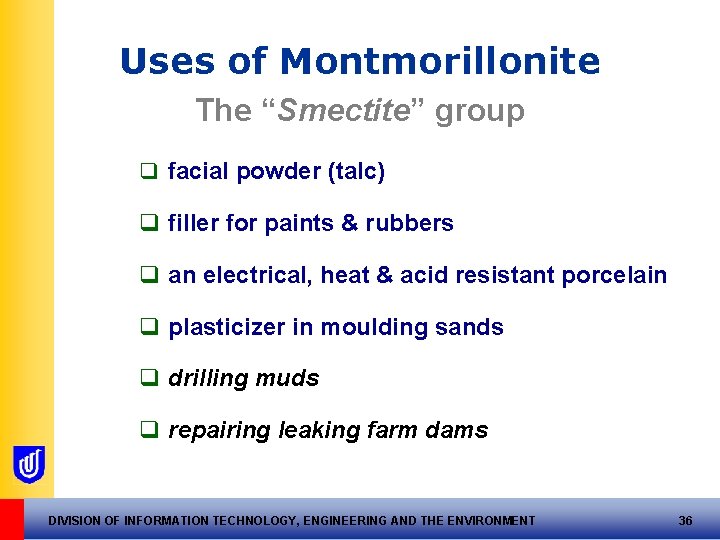
Uses of Montmorillonite The “Smectite” group q facial powder (talc) q filler for paints & rubbers q an electrical, heat & acid resistant porcelain q plasticizer in moulding sands q drilling muds q repairing leaking farm dams DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 36

In Summary 1. The basic building blocks of clays are small 2. Si, O, H and Al are the chief ingredients 3. Tetrahedral & octahedral sheets possible 4. Different combinations of sheets form the basic micelles of clay minerals 5. Clay mineral properties vary due to the nature of bonding of the sheets between micelles DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 37

Revision • What is a clay micelle? • Describe how a 1: 1 clay mineral is formed • How does the Mica group of clay minerals differ from the 1: 1 clay minerals? DIVISION OF INFORMATION TECHNOLOGY, ENGINEERING AND THE ENVIRONMENT 38