Introduction History of science Mendelian inheritance 2020 Gregor


























- Slides: 80

Introduction History of science Mendelian inheritance 2020.

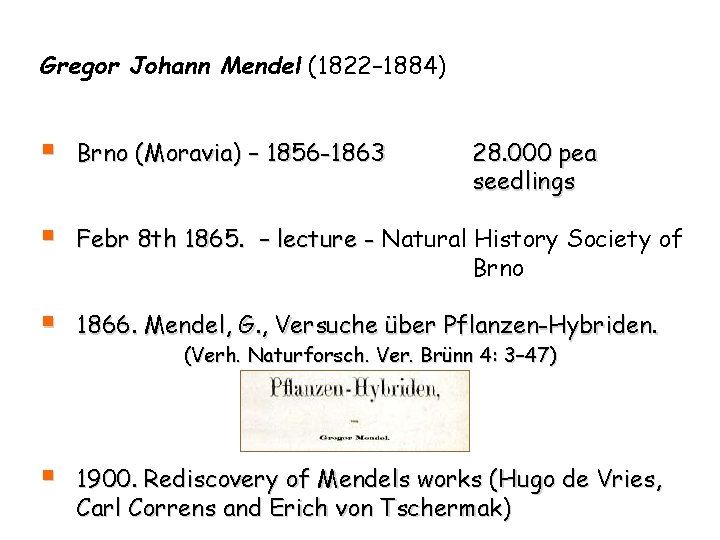
Gregor Johann Mendel (1822– 1884) § Brno (Moravia) – 1856 -1863 § Febr 8 th 1865. – lecture - Natural History Society of Brno § 1866. Mendel, G. , Versuche über Pflanzen-Hybriden. § 1900. Rediscovery of Mendels works (Hugo de Vries, Carl Correns and Erich von Tschermak) 28. 000 pea seedlings (Verh. Naturforsch. Ver. Brünn 4: 3– 47)

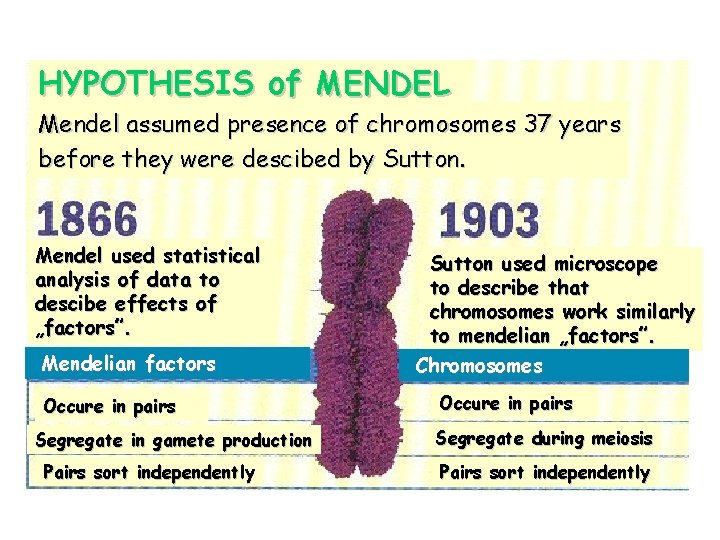
When Mendel described his „factors” … Chromosomes ! ! ! d e b i r c s Genes e d T O N e r e W DNA

HYPOTHESIS of MENDEL Mendel assumed presence of chromosomes 37 years before they were descibed by Sutton. Mendel used statistical analysis of data to descibe effects of „factors”. Mendelian factors Occure in pairs Segregate in gamete production Pairs sort independently Sutton used microscope to describe that chromosomes work similarly to mendelian „factors”. Chromosomes Occure in pairs Segregate during meiosis Pairs sort independently

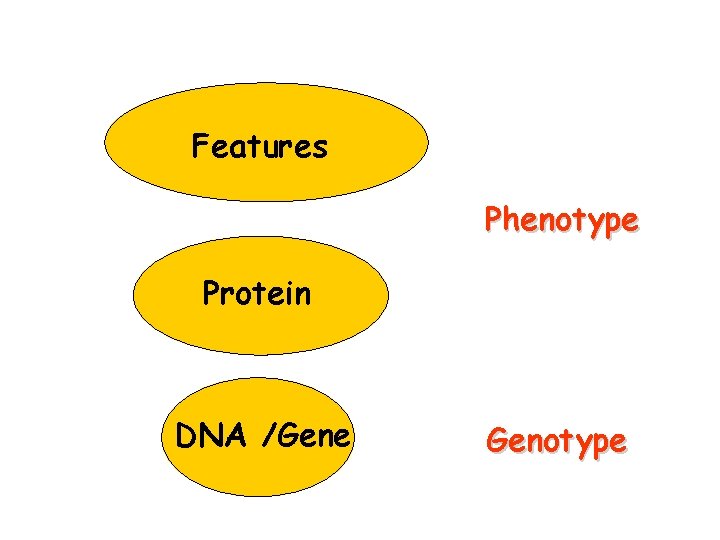
Features Phenotype Protein DNA /Gene Genotype

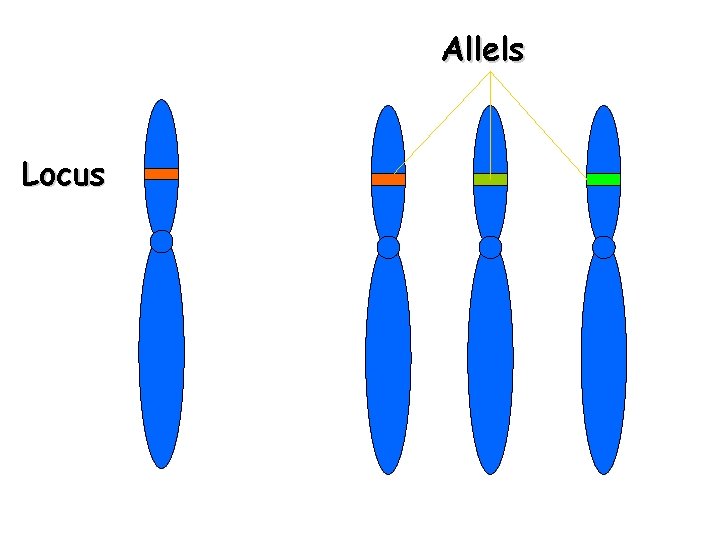
Allels Locus

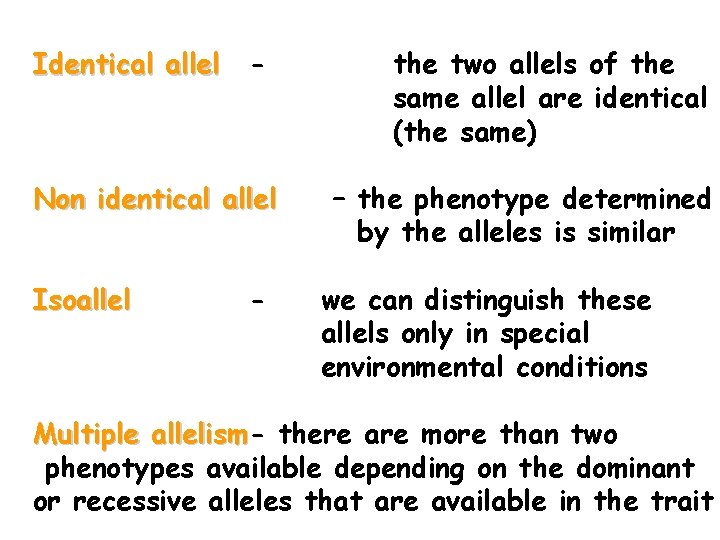
Identical allel - Non identical allel Isoallel - the two allels of the same allel are identical (the same) – the phenotype determined by the alleles is similar we can distinguish these allels only in special environmental conditions Multiple allelism there are more than two phenotypes available depending on the dominant or recessive alleles that are available in the trait

Homozygote Heterozygote X Y Hemyzygote

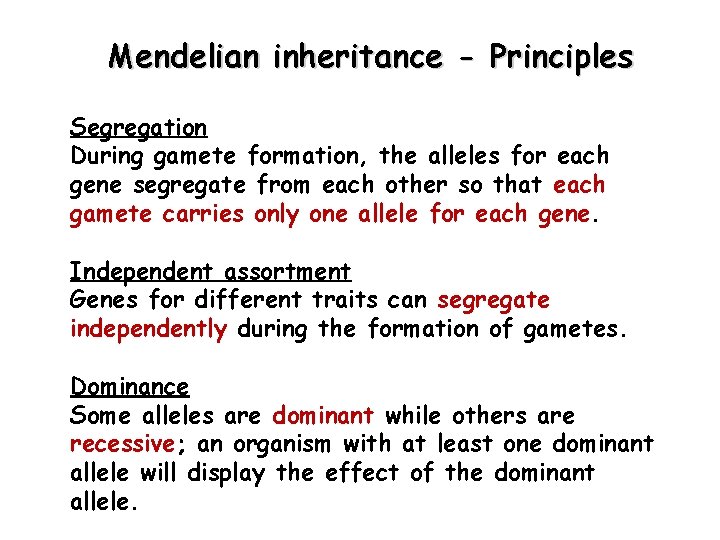
Mendelian inheritance - Principles Segregation During gamete formation, the alleles for each gene segregate from each other so that each gamete carries only one allele for each gene. Independent assortment Genes for different traits can segregate independently during the formation of gametes. Dominance Some alleles are dominant while others are recessive; an organism with at least one dominant allele will display the effect of the dominant allele.

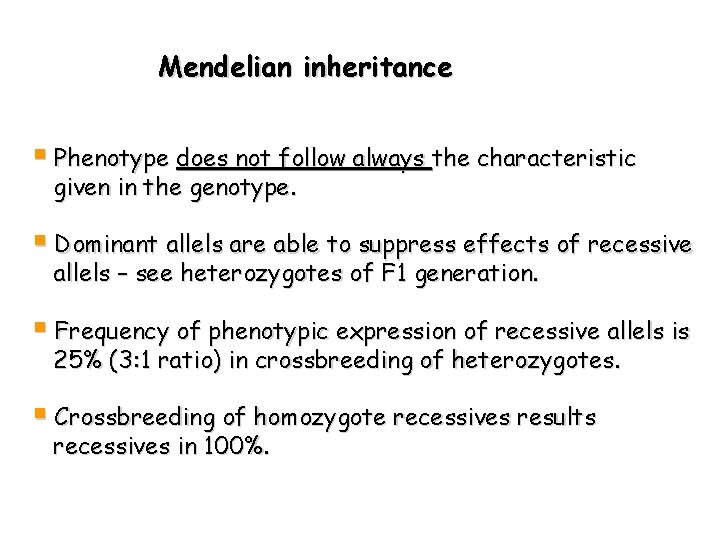
Mendelian inheritance § Phenotype does not follow always the characteristic given in the genotype. § Dominant allels are able to suppress effects of recessive allels – see heterozygotes of F 1 generation. § Frequency of phenotypic expression of recessive allels is 25% (3: 1 ratio) in crossbreeding of heterozygotes. § Crossbreeding of homozygote recessives results recessives in 100%.

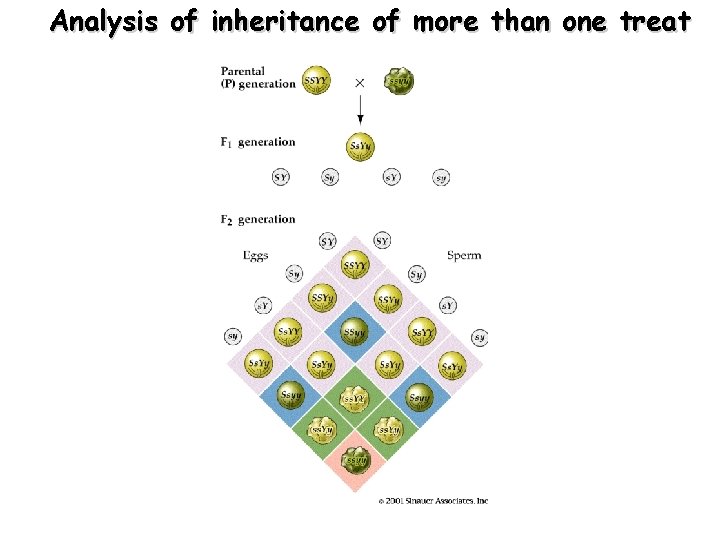
Analysis of inheritance of more than one treat

Mendels Principle-3 - Independent assortment. It is valid with certain restrictions.

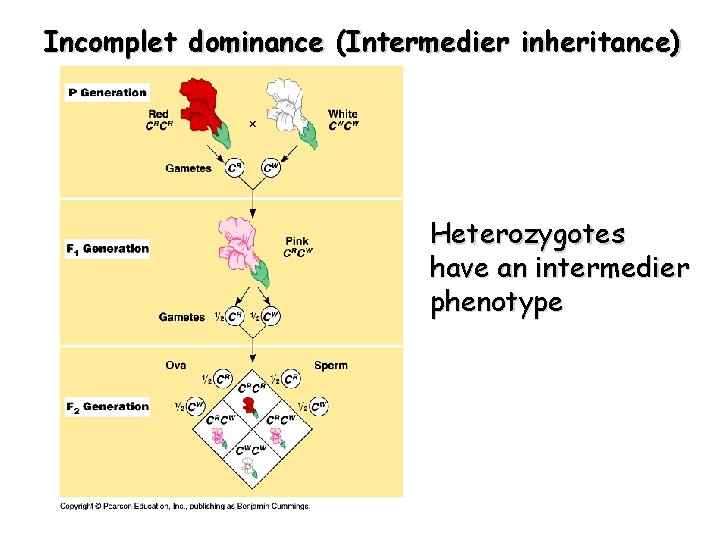
Incomplet dominance (Intermedier inheritance) Heterozygotes have an intermedier phenotype


Autosomal monogenic inheritance Dr. habil. Kohidai Laszlo Department of Genetics, Cell- and Immunobiology Semmelweis University Budapest /2020/

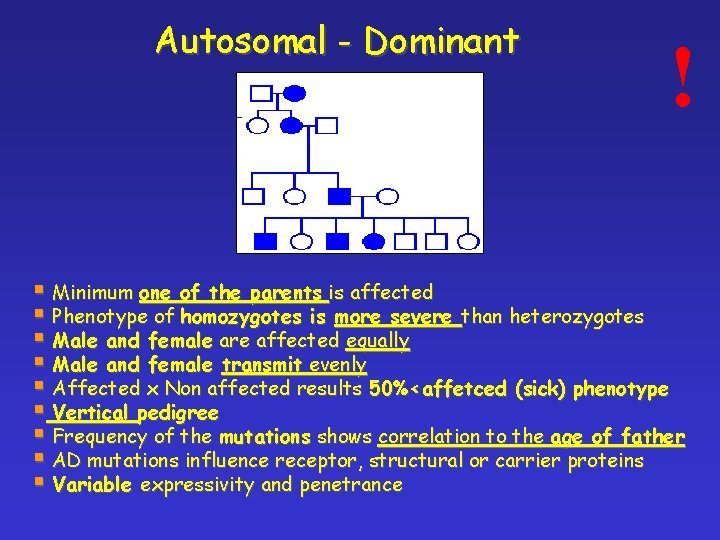
Autosomal - Dominant ! § Minimum one of the parents is affected § Phenotype of homozygotes is more severe than heterozygotes § Male and female are affected equally § Male and female transmit evenly § Affected x Non affected results 50%<affetced (sick) phenotype § Vertical pedigree § Frequency of the mutations shows correlation to the age of father § AD mutations influence receptor, structural or carrier proteins § Variable expressivity and penetrance

Dominant autosomal § ~ 2200 known dominant trait § frequency 0. 1 -3/1000/birth § most frequently affected organs: skeleton central nerve system

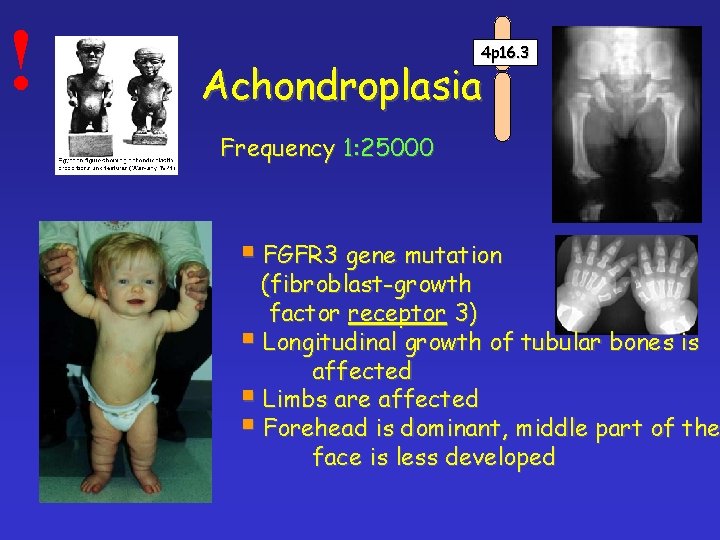
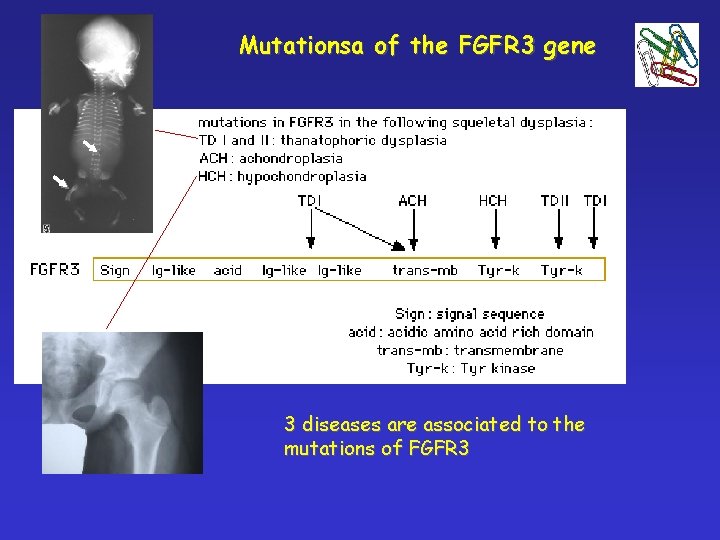
! 4 p 16. 3 Achondroplasia Frequency 1: 25000 § FGFR 3 gene mutation (fibroblast-growth factor receptor 3) § Longitudinal growth of tubular bones is affected § Limbs are affected § Forehead is dominant, middle part of the face is less developed

Achondroplasia

Rhinoceros unicornis Teleoceras fossiger sheeps

FGFR 3 gene § § locus: 4 p 16. 3 DNA: 16. 5 Kb; 19 exon; exon 1 is not known in human RNA: 4. 0 Kb m. RNS; alternative splicing Exons 7 and 8: two m. RNA isoforms IIIb and IIIc Expressed in: brain, cartilage, liver, kidney, inner ear

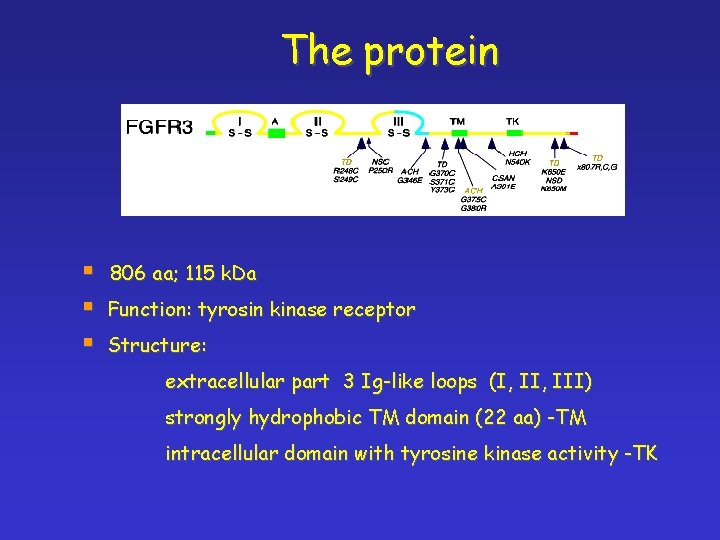
The protein § § § 806 aa; 115 k. Da Function: tyrosin kinase receptor Structure: extracellular part 3 Ig-like loops (I, III) strongly hydrophobic TM domain (22 aa) -TM intracellular domain with tyrosine kinase activity -TK

Mutationsa of the FGFR 3 gene 3 diseases are associated to the mutations of FGFR 3

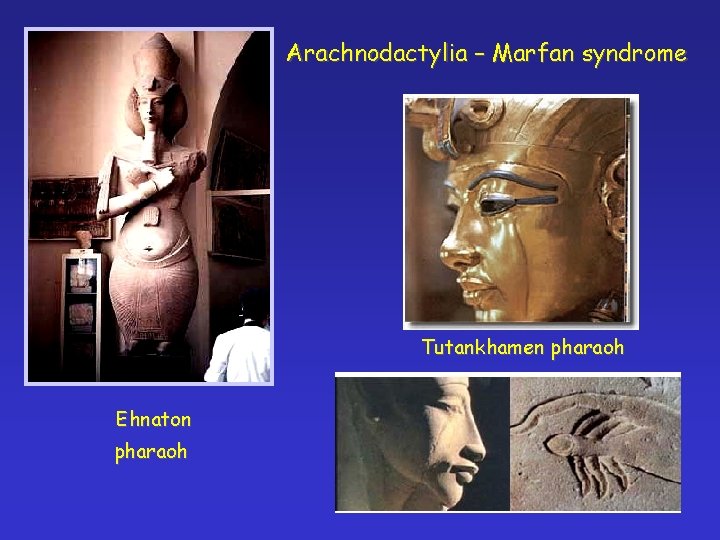
Arachnodactylia – Marfan syndrome Antoine Bernard-Jean Marfan (1896) Gabrielle

Arachnodactylia – Marfan syndrome Tutankhamen pharaoh Ehnaton pharaoh

Mary of Scotland Abraham Lincoln

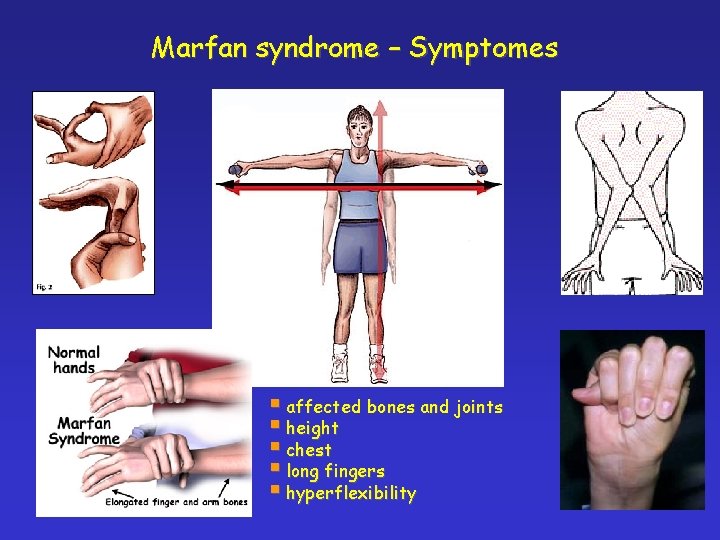
Marfan syndrome – Symptomes § affected bones and joints § height § chest § long fingers § hyperflexibility

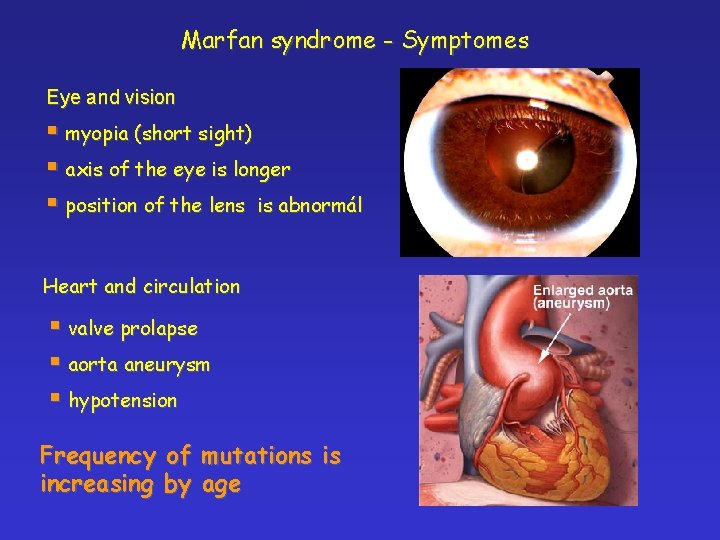
Marfan syndrome - Symptomes Eye and vision § myopia (short sight) § axis of the eye is longer § position of the lens is abnormál Heart and circulation § valve prolapse § aorta aneurysm § hypotension Frequency of mutations is increasing by age

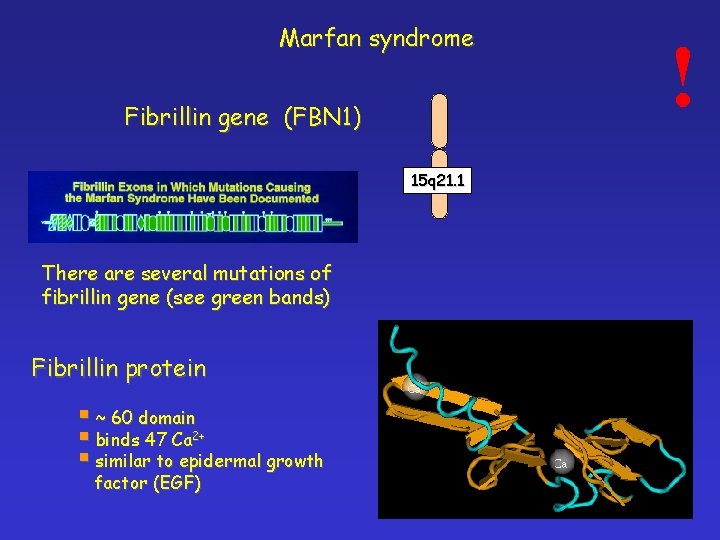
Marfan syndrome Fibrillin gene (FBN 1) 15 q 21. 1 There are several mutations of fibrillin gene (see green bands) Fibrillin protein § ~ 60 domain § binds 47 Ca 2+ § similar to epidermal growth factor (EGF) !

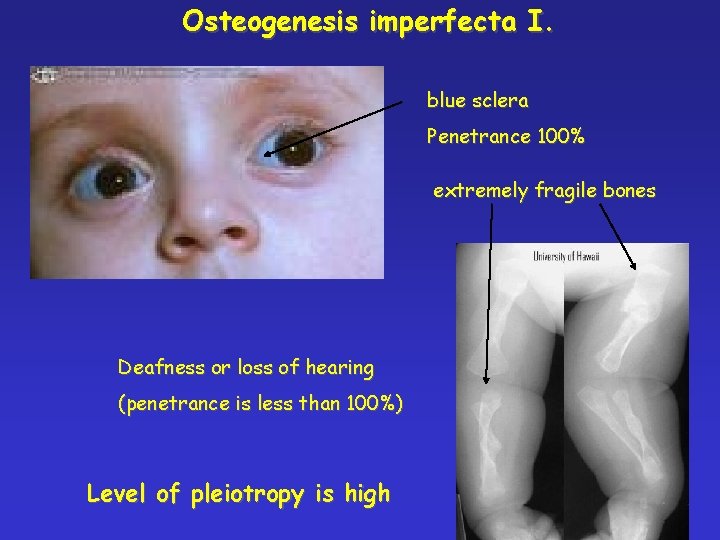
Osteogenesis imperfecta I. blue sclera Penetrance 100% extremely fragile bones Deafness or loss of hearing (penetrance is less than 100%) Level of pleiotropy is high

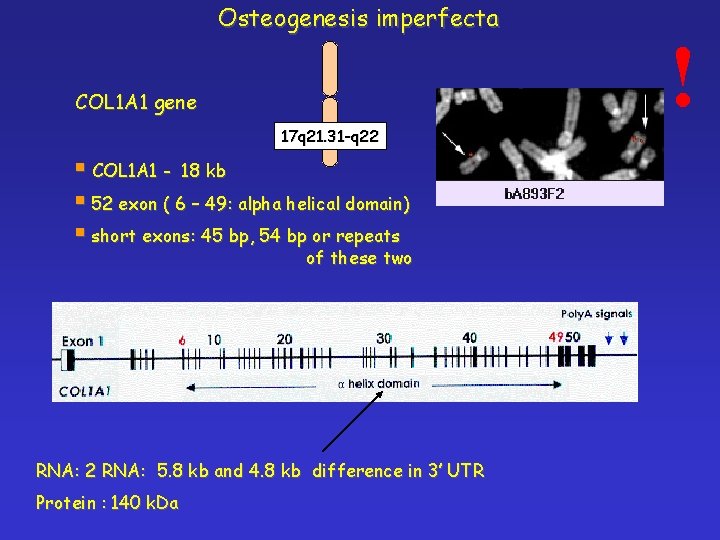
Osteogenesis imperfecta COL 1 A 1 gene 17 q 21. 31 -q 22 § COL 1 A 1 - 18 kb § 52 exon ( 6 – 49: alpha helical domain) § short exons: 45 bp, 54 bp or repeats of these two RNA: 2 RNA: 5. 8 kb and 4. 8 kb difference in 3’ UTR Protein : 140 k. Da !

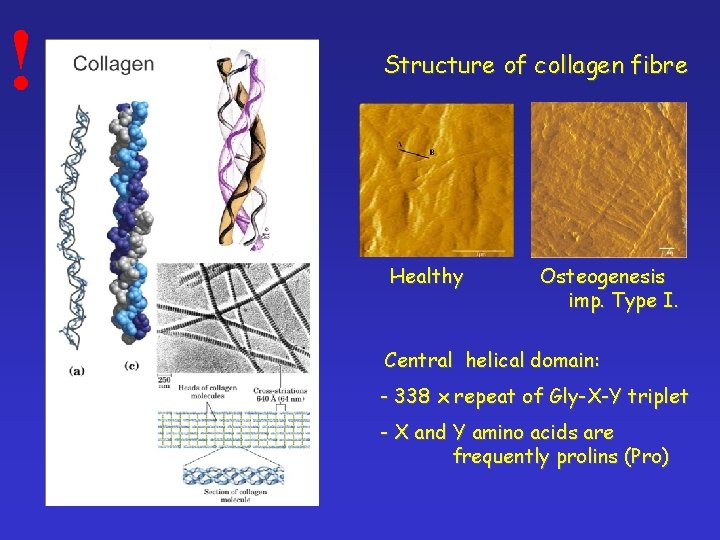
! Structure of collagen fibre Healthy Osteogenesis imp. Type I. Central helical domain: - 338 x repeat of Gly-X-Y triplet - X and Y amino acids are frequently prolins (Pro)

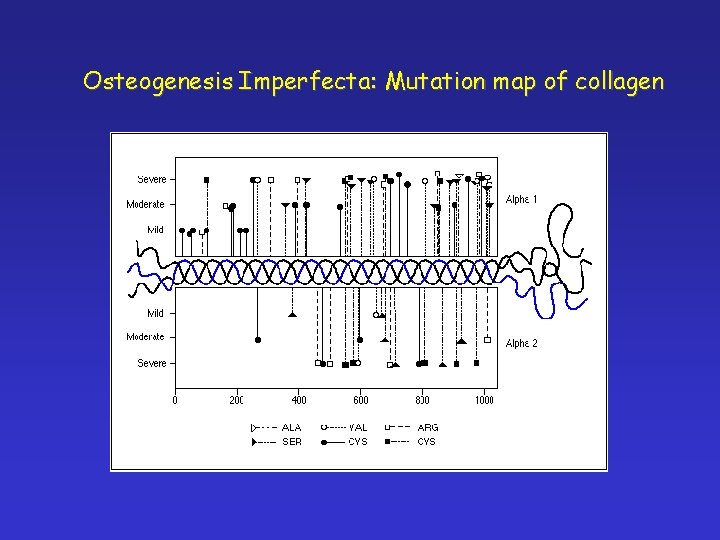
Osteogenesis Imperfecta: Mutation map of collagen

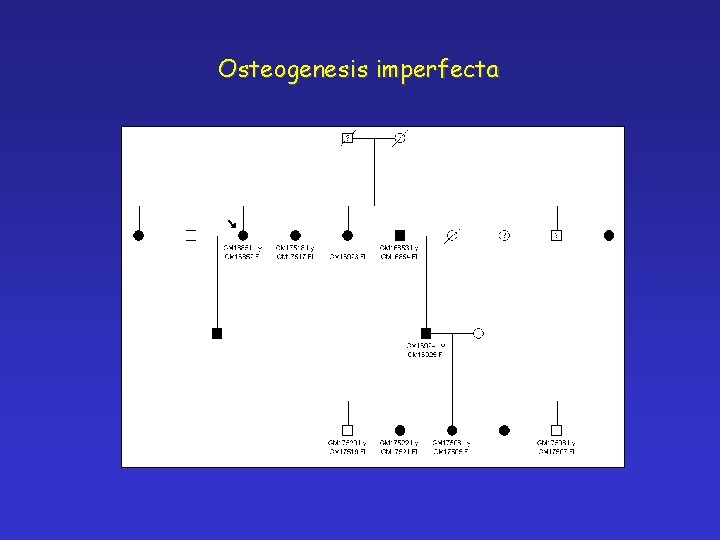
Osteogenesis imperfecta

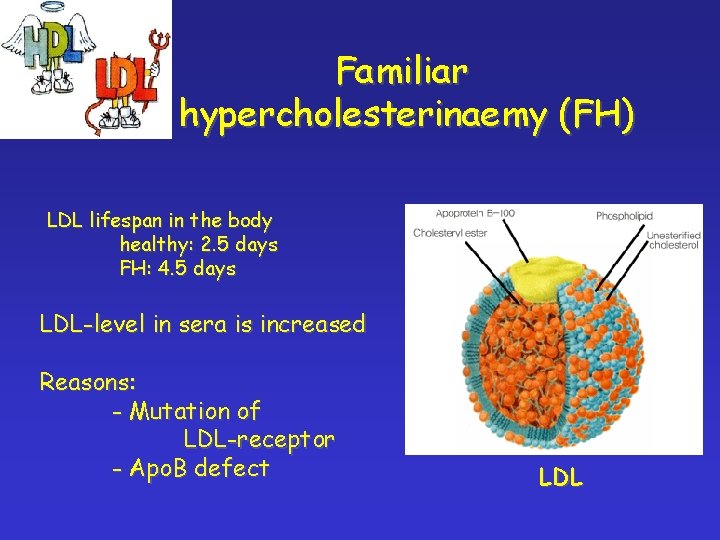
Familiar hypercholesterinaemy Main clinical symptoms: - early onset of cardial and circulatory system diseases (myocardial innfarction, vascular diseases of brain and peripherial blood vessels) - xanthoma - diseases of the eye

Familiar hypercholesterinaemy (FH) LDL lifespan in the body healthy: 2. 5 days FH: 4. 5 days LDL-level in sera is increased Reasons: - Mutation of LDL-receptor - Apo. B defect LDL


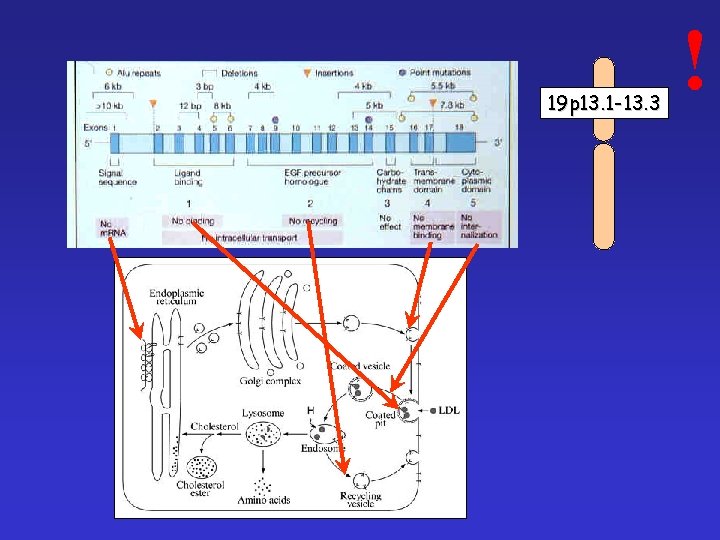
19 p 13. 1 -13. 3 !

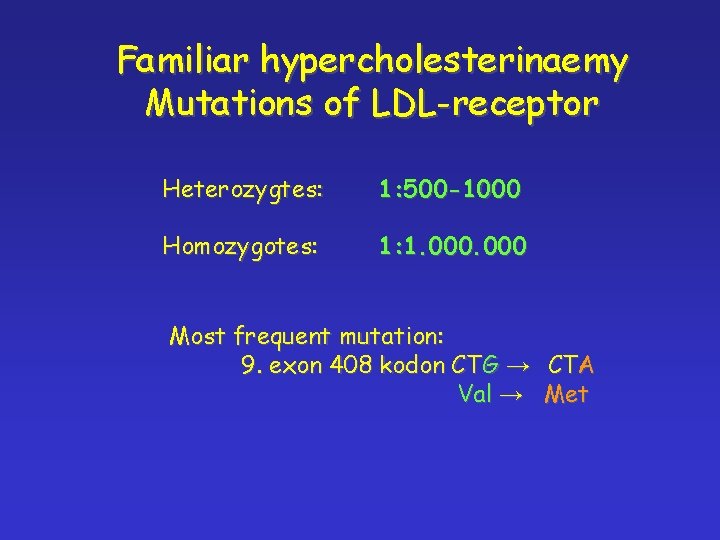
Familiar hypercholesterinaemy Mutations of LDL-receptor Heterozygtes: 1: 500 -1000 Homozygotes: 1: 1. 000 Most frequent mutation: 9. exon 408 kodon CTG → CTA Val → Met

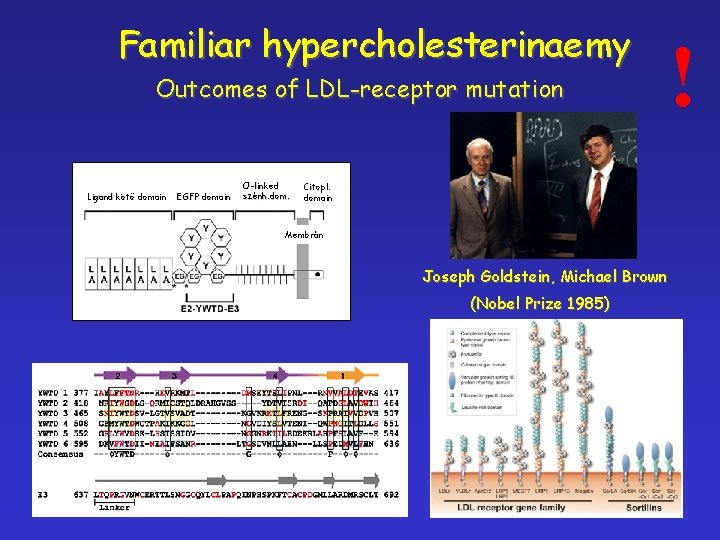
Familiar hypercholesterinaemy Outcomes of LDL-receptor mutation Ligand kötő domain EGFP domain O-linked szénh. dom. Citopl. domain Membrán Joseph Goldstein, Michael Brown (Nobel Prize 1985) !

Trinucleotide-repeat diseases !

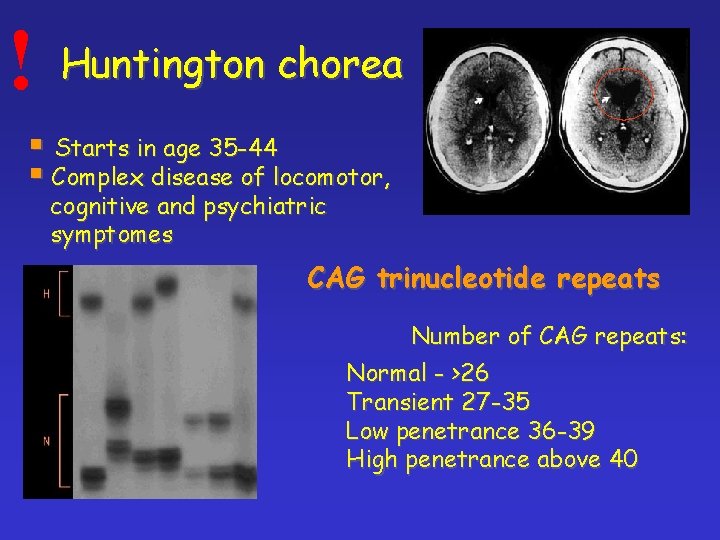
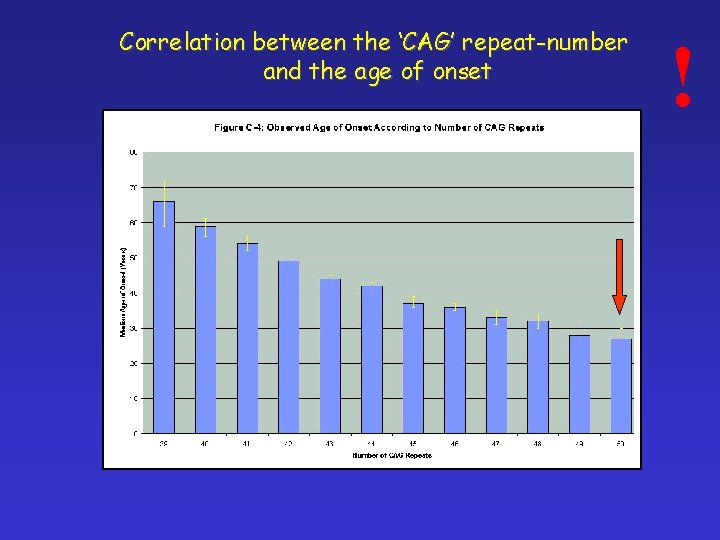
! Huntington chorea § Starts in age 35 -44 § Complex disease of locomotor, cognitive and psychiatric symptomes CAG trinucleotide repeats Number of CAG repeats: Normal - >26 Transient 27 -35 Low penetrance 36 -39 High penetrance above 40

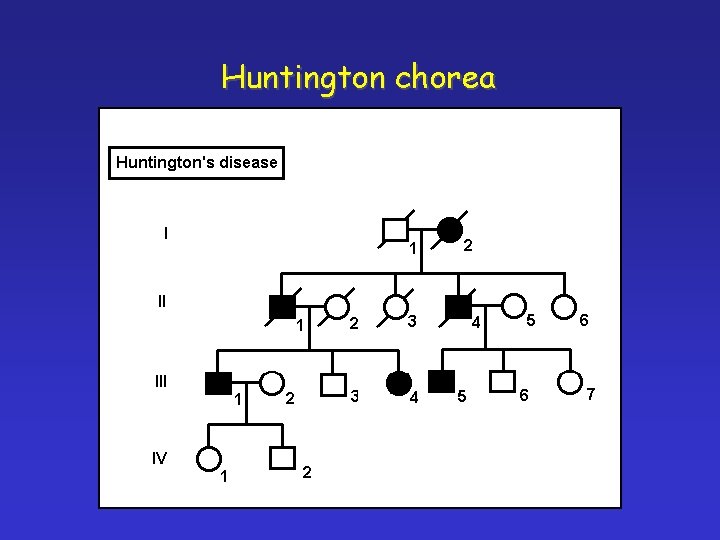
Huntington chorea

Huntington chorea - (CAGn) 4 § Gain-of-function mutation § The function of the Huntingtin gene in human is not known !

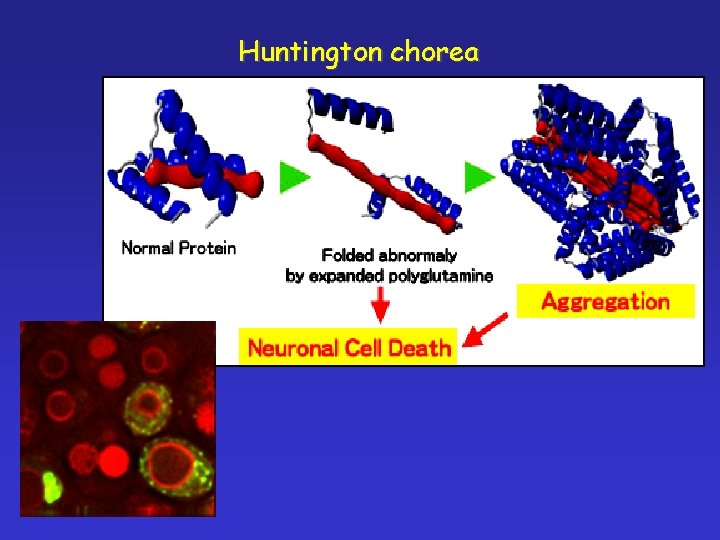
Huntington chorea

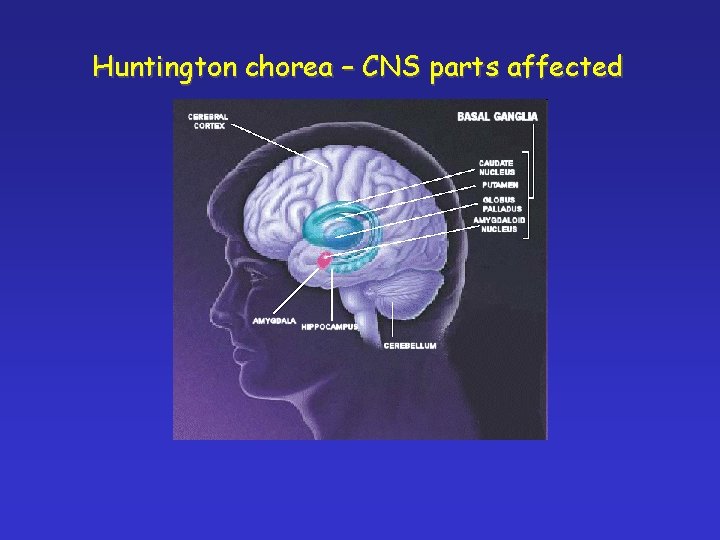
Huntington chorea – CNS parts affected

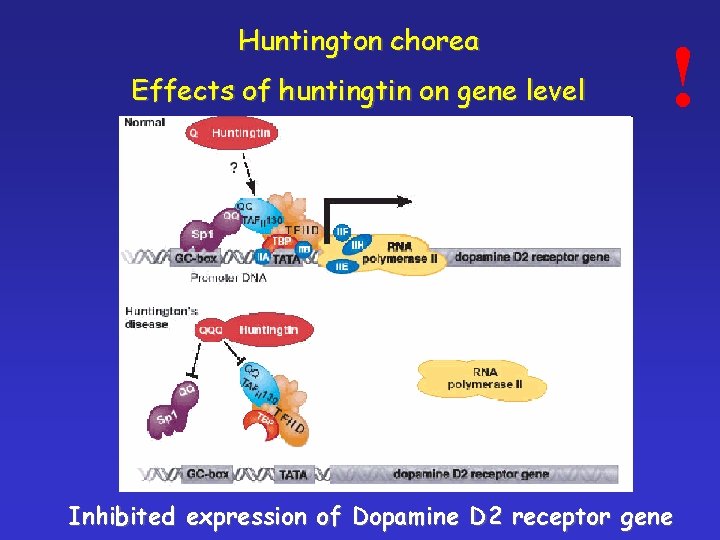
Huntington chorea Effects of huntingtin on gene level ! Inhibited expression of Dopamine D 2 receptor gene

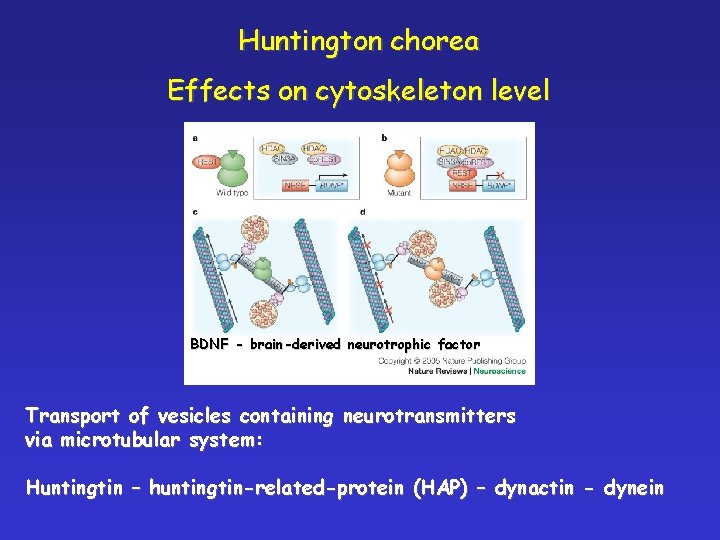
Huntington chorea Effects on cytoskeleton level BDNF - brain-derived neurotrophic factor Transport of vesicles containing neurotransmitters via microtubular system: Huntingtin – huntingtin-related-protein (HAP) – dynactin - dynein

Correlation between the ‘CAG’ repeat-number and the age of onset !

§ George Huntington (1850 -1916) § Grandfather and father were farmer doctors – their anamnestic files supported Huntington to describe the disease § The disease was described in 1872 § Medical and Surgical Reporter of Philadelphia chorea = maniac dance

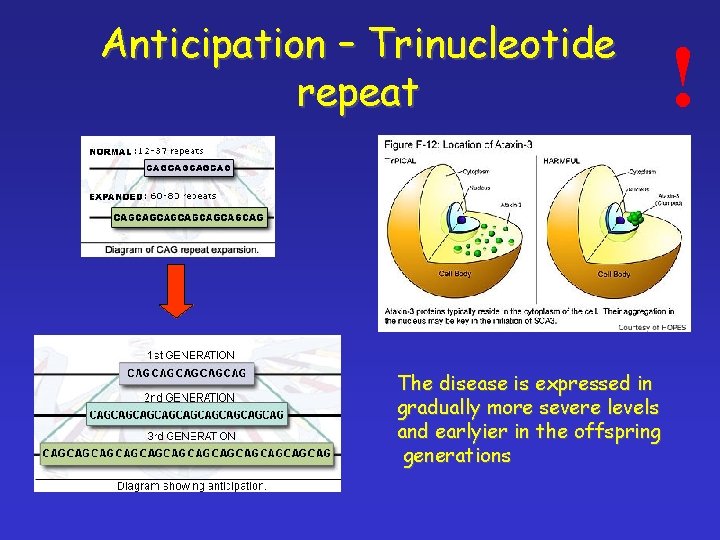
Anticipation – Trinucleotide repeat The disease is expressed in gradually more severe levels and earlyier in the offspring generations !

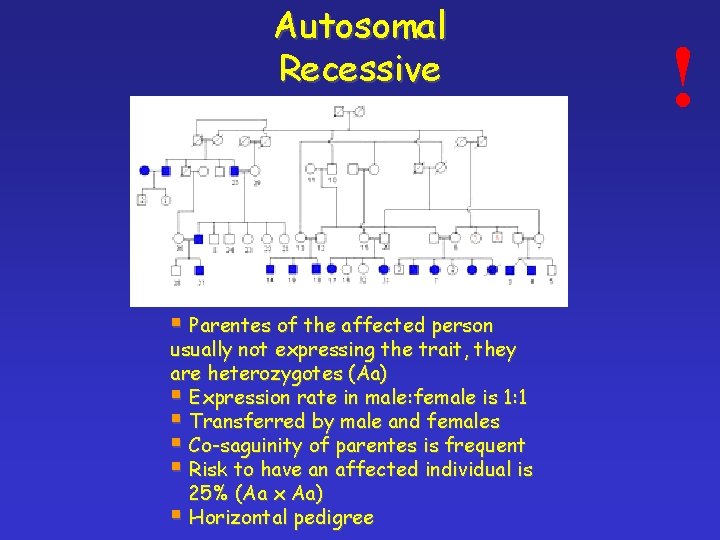
Autosomal Recessive § Parentes of the affected person usually not expressing the trait, they are heterozygotes (Aa) § Expression rate in male: female is 1: 1 § Transferred by male and females § Co-saguinity of parentes is frequent § Risk to have an affected individual is 25% (Aa x Aa) § Horizontal pedigree !

Recessive autosomal § 1700 known human recessive traits § More than 15% is enzymopathy (e. g. phenylalanine hydroxylase, hexose aminidase) § Mutations of haemoglobin Multiple allelism – several mutations of one gene are responsible for the development of a symptome Complex heterozygote – an individual who possess two diverse, mutant allels of a gene E. g. Cystic fibrosis – 850 different mutations

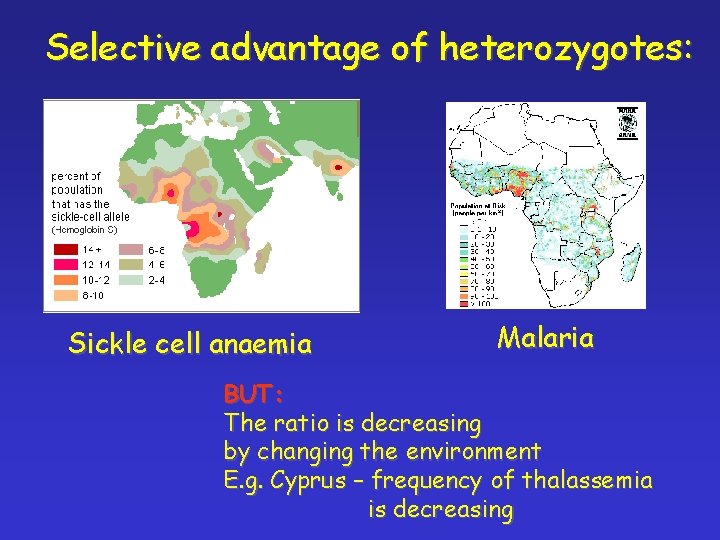
Frequency of recessive diseases There is a significant difference in diverse ethnic groups REASON: reproductive advantage of heterozygotes to homozygotes ENVIRONMENTAL FACTORS Selective advantages of heterozygotes Other reasons: directed marriages in some ethnic groups

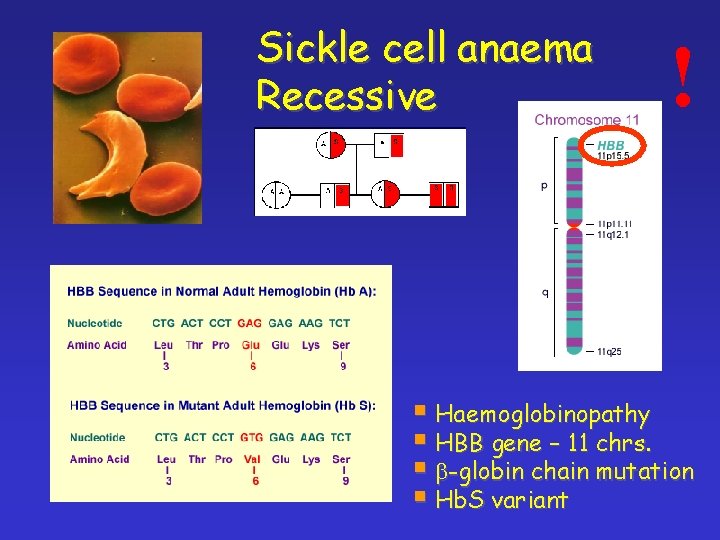
Sickle cell anaema Recessive ! § Haemoglobinopathy § HBB gene – 11 chrs. § b-globin chain mutation § Hb. S variant

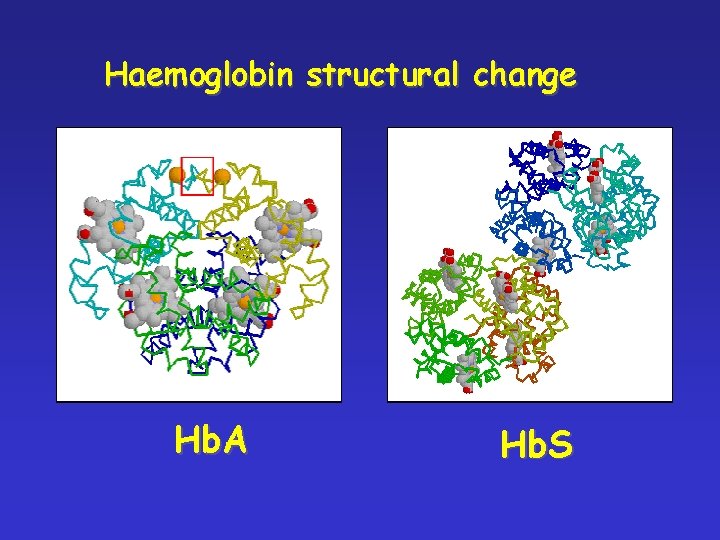
Haemoglobin structural change Hb. A Hb. S

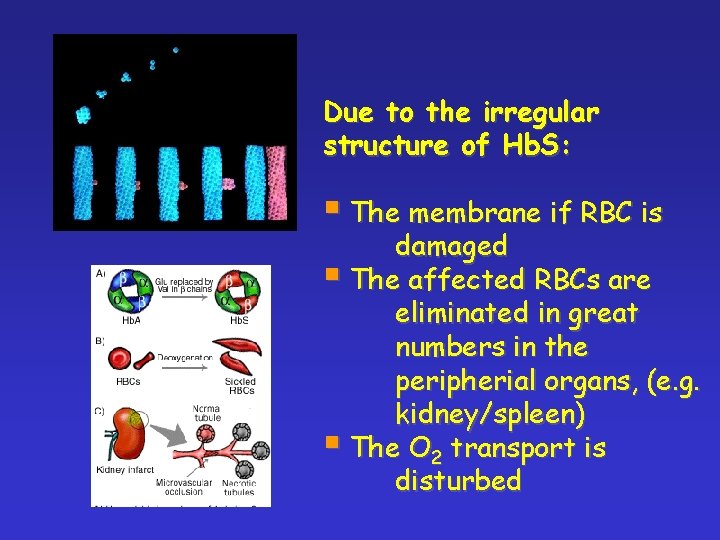
Due to the irregular structure of Hb. S: § The membrane if RBC is damaged § The affected RBCs are eliminated in great numbers in the peripherial organs, (e. g. kidney/spleen) § The O 2 transport is disturbed


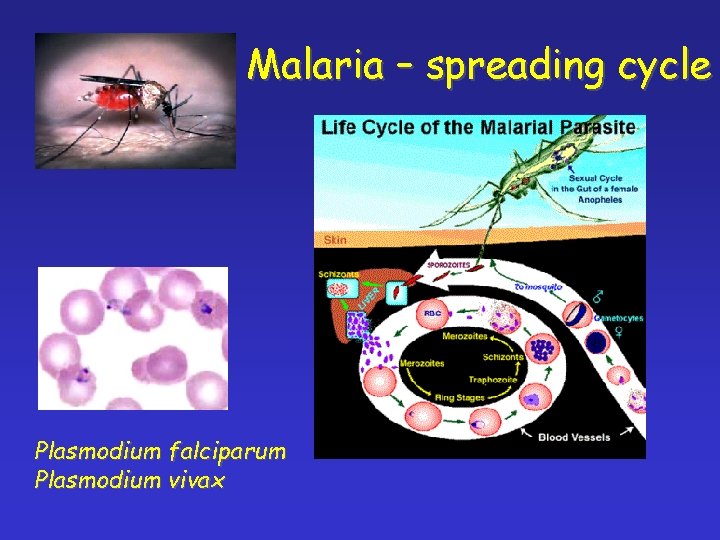
Malaria – spreading cycle Plasmodium falciparum Plasmodium vivax

Selective advantage of heterozygotes: Sickle cell anaemy Malaria !

Selective advantage of heterozygotes: Sickle cell anaemia Malaria BUT: The ratio is decreasing by changing the environment E. g. Cyprus – frequency of thalassemia is decreasing

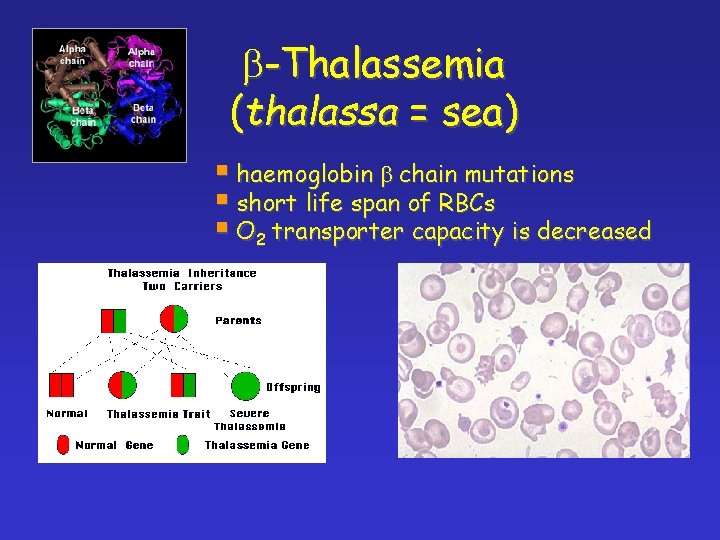
b-Thalassemia (thalassa = sea)

b-Thalassemia (thalassa = sea) § haemoglobin b chain mutations § short life span of RBCs § O 2 transporter capacity is decreased

Cystic fibrosis

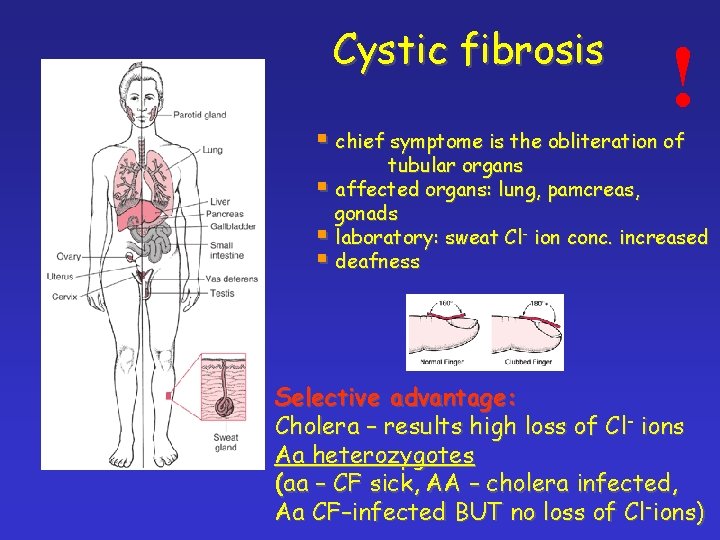
Cystic fibrosis ! § chief symptome is the obliteration of tubular organs § affected organs: lung, pamcreas, gonads § laboratory: sweat Cl- ion conc. increased § deafness Selective advantage: Cholera – results high loss of Cl- ions Aa heterozygotes (aa – CF sick, AA – cholera infected, Aa CF–infected BUT no loss of Cl-ions)

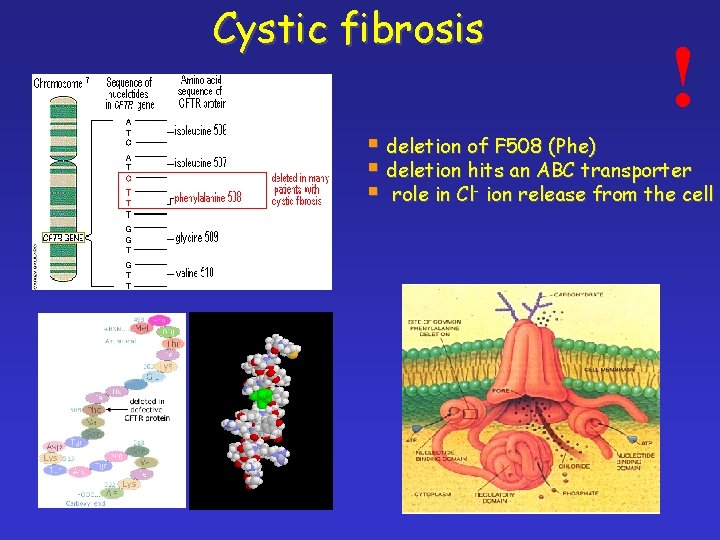
Cystic fibrosis ! § deletion of F 508 (Phe) § deletion hits an ABC transporter § role in Cl- ion release from the cell

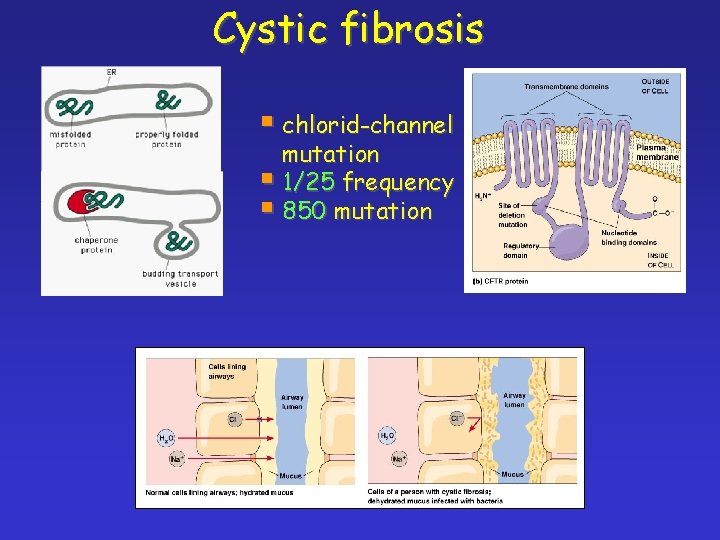
Cystic fibrosis § chlorid-channel mutation § 1/25 frequency § 850 mutation

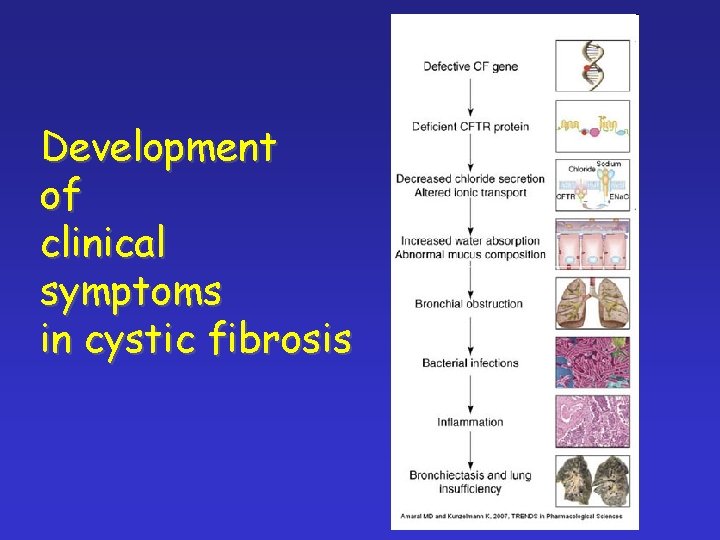
Development of clinical symptoms in cystic fibrosis

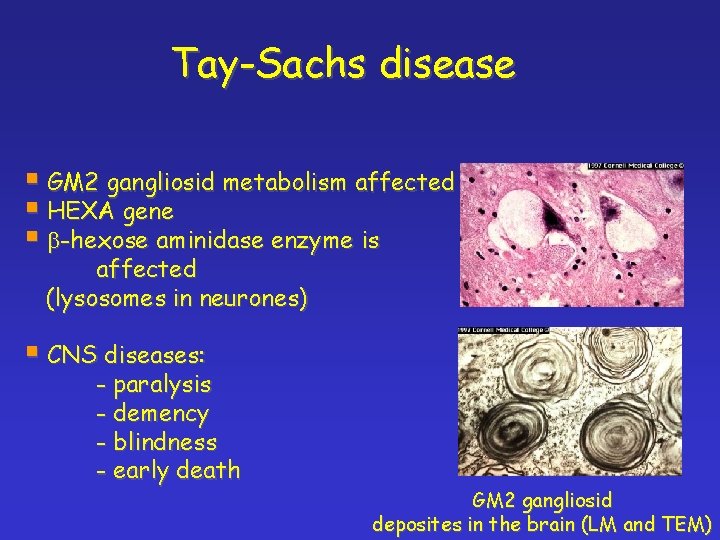
Tay-Sachs disease § GM 2 gangliosid metabolism affected § HEXA gene § b-hexose aminidase enzyme is affected (lysosomes in neurones) § CNS diseases: - paralysis - demency - blindness - early death GM 2 gangliosid deposites in the brain (LM and TEM)

! Tay-Sachs disease Lysosome membrane phospholipid GM 2 activator GM 3 GM 2 Hex A

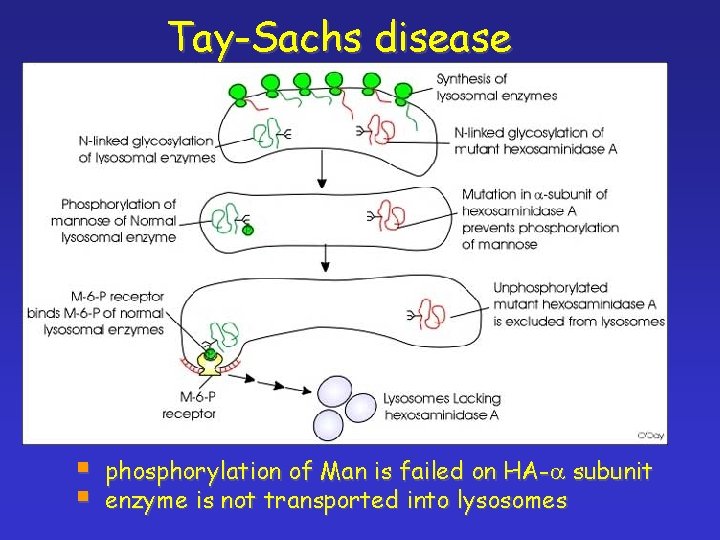
Tay-Sachs disease § § phosphorylation of Man is failed on HA-a subunit enzyme is not transported into lysosomes

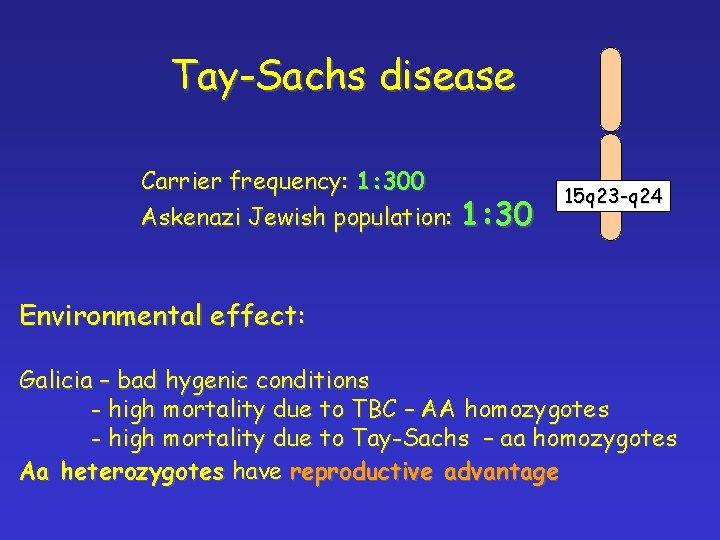
Tay-Sachs disease Carrier frequency: 1: 300 Askenazi Jewish population: 1: 30 15 q 23 -q 24 Environmental effect: Galicia – bad hygenic conditions - high mortality due to TBC – AA homozygotes - high mortality due to Tay-Sachs – aa homozygotes Aa heterozygotes have reproductive advantage

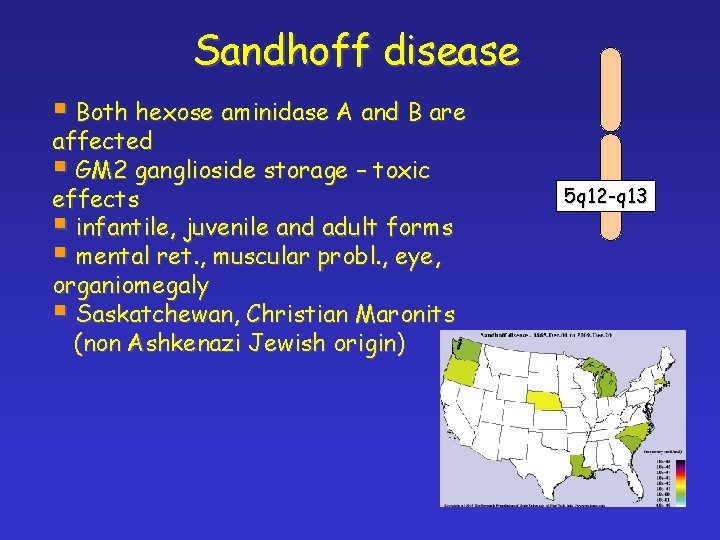
Sandhoff disease § Both hexose aminidase A and B are affected § GM 2 ganglioside storage – toxic effects § infantile, juvenile and adult forms § mental ret. , muscular probl. , eye, organiomegaly § Saskatchewan, Christian Maronits (non Ashkenazi Jewish origin) 5 q 12 -q 13

! Other monogenic recessive autosomal diseases

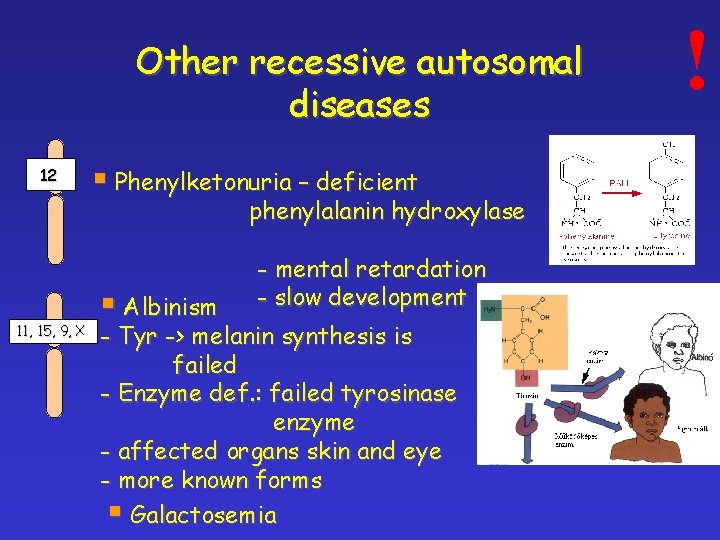
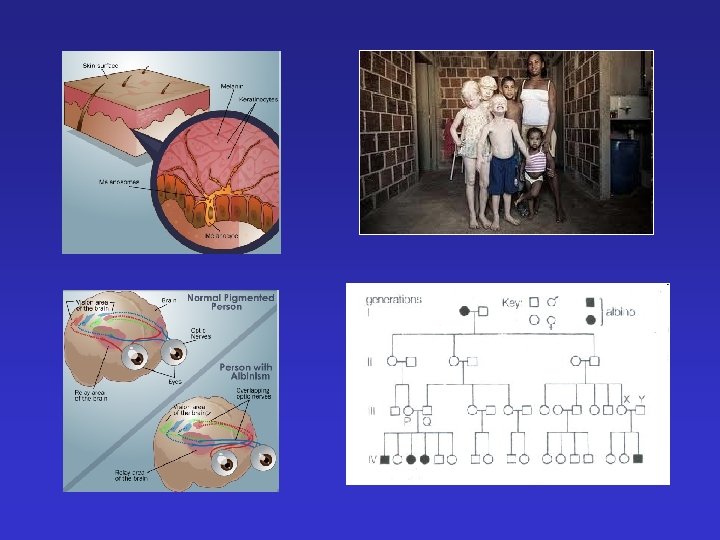
Other recessive autosomal diseases 12 § Phenylketonuria – deficient phenylalanin hydroxylase 11, 15, 9, X § Albinism - mental retardation - slow development - Tyr -> melanin synthesis is failed - Enzyme def. : failed tyrosinase enzyme - affected organs skin and eye - more known forms § Galactosemia !


Albino and normal kangaroos Albino Barking Deer Albino crab Albino dingo

Effect of ethnic diferences on the expression of inherited diseases


 Family resemblance test
Family resemblance test Non mendelian inheritance
Non mendelian inheritance Cystic fibrosis mendelian inheritance
Cystic fibrosis mendelian inheritance Probability laws govern mendelian inheritance
Probability laws govern mendelian inheritance Mendelian pattern of inheritance
Mendelian pattern of inheritance 3 laws of inheritance
3 laws of inheritance Who was mendal
Who was mendal Mendel's experimental design
Mendel's experimental design Mendelian traits
Mendelian traits Inheritance definition computer science
Inheritance definition computer science What are your favorite subjects?
What are your favorite subjects? Introduction to inheritance
Introduction to inheritance Science fusion think central
Science fusion think central Blood type matrix
Blood type matrix Non mendelian genetics multiple alleles
Non mendelian genetics multiple alleles Drug
Drug Section 11-3 exploring mendelian genetics answer key
Section 11-3 exploring mendelian genetics answer key Extending mendelian genetics chapter 7
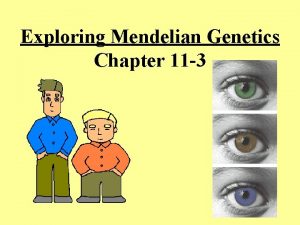
Extending mendelian genetics chapter 7 Section 11-3 exploring mendelian genetics
Section 11-3 exploring mendelian genetics Chapter 10 section 2: mendelian genetics
Chapter 10 section 2: mendelian genetics Mendel was a
Mendel was a 11.3 exploring mendelian genetics
11.3 exploring mendelian genetics 11.3 exploring mendelian genetics
11.3 exploring mendelian genetics Non mendelian law
Non mendelian law Non mendelian law
Non mendelian law Karyotype
Karyotype Mendelian traits
Mendelian traits Extension of mendelian genetics
Extension of mendelian genetics Section 11-3 exploring mendelian genetics answer key
Section 11-3 exploring mendelian genetics answer key Xnxn horse
Xnxn horse Chapter 7 extending mendelian genetics vocabulary practice
Chapter 7 extending mendelian genetics vocabulary practice Pewarisan sifat non mendelian
Pewarisan sifat non mendelian Penetrance
Penetrance Codominance
Codominance Chapter 10 sexual reproduction and genetics
Chapter 10 sexual reproduction and genetics Difference between mendelian and chromosomal disorders
Difference between mendelian and chromosomal disorders Mendel's law
Mendel's law Mendel
Mendel Mendelian genetics vocab
Mendelian genetics vocab Dominant epistasis example
Dominant epistasis example Autosomal dihybrid cross
Autosomal dihybrid cross Mendelian traits
Mendelian traits Incomplete vs codominance
Incomplete vs codominance Punnett square color blindness
Punnett square color blindness Mendelian genetics concept map
Mendelian genetics concept map Mendelian genetics vocabulary
Mendelian genetics vocabulary Mendelian
Mendelian H antigen
H antigen Eslkidstuff
Eslkidstuff Who is gregor mendel and what is he famous for
Who is gregor mendel and what is he famous for Why did mendel prevent his plants from self-pollinating?
Why did mendel prevent his plants from self-pollinating? Gregor mohorcic
Gregor mohorcic Who was mendal
Who was mendal Austrian genetic traits
Austrian genetic traits Father of genetics
Father of genetics Rryy x rryy
Rryy x rryy La metamorfosis grete samsa
La metamorfosis grete samsa Gregor mendal
Gregor mendal Gregor mendel chart
Gregor mendel chart Chapter 12 lesson 1 the work of gregor mendel
Chapter 12 lesson 1 the work of gregor mendel Suit separate
Suit separate Gregor gabriel
Gregor gabriel What did gregor mendel research
What did gregor mendel research Who is gregor mendel
Who is gregor mendel What did gregor mendel do
What did gregor mendel do Gregor mendel conclusions
Gregor mendel conclusions Chapter 12 lesson 1 the work of gregor mendel
Chapter 12 lesson 1 the work of gregor mendel Gregor kiczales ubc
Gregor kiczales ubc Gregor mendel’s principles of genetics apply to
Gregor mendel’s principles of genetics apply to Apolinea
Apolinea Gregor guron
Gregor guron 11-2 probability and punnett squares answer key
11-2 probability and punnett squares answer key Gregor samsa awoke
Gregor samsa awoke Jozef gregor tajovsky najznamejsie diela
Jozef gregor tajovsky najznamejsie diela The metamorphosis activities
The metamorphosis activities Gregor mendel
Gregor mendel Gregor mendel summary
Gregor mendel summary Johann sebastian bach geboren
Johann sebastian bach geboren Law of segregation meiosis
Law of segregation meiosis Gregor kiczales
Gregor kiczales Gregor mendol
Gregor mendol