International Symposium on Molecular Spectroscopy 69 th Meeting




- Slides: 15

International Symposium on Molecular Spectroscopy, 69 th Meeting Champaign-Urbana, June 16 -20, 2014 Deuterated water hexamer observed by chirped-pulse rotational spectroscopy L. Evangelisti, C. Perez, S. Lobsiger, N. A. Seifert, D. P. Zaleski, B. H. Pate Department of Chemistry, University of Virginia Z. Kisiel Institute of Physics, Polish Academy of Sciences. Poland. B. Temelso, G. C. Shields Dean’s Office, College of Arts and Sciences, and Department of Chemistry, Bucknell University

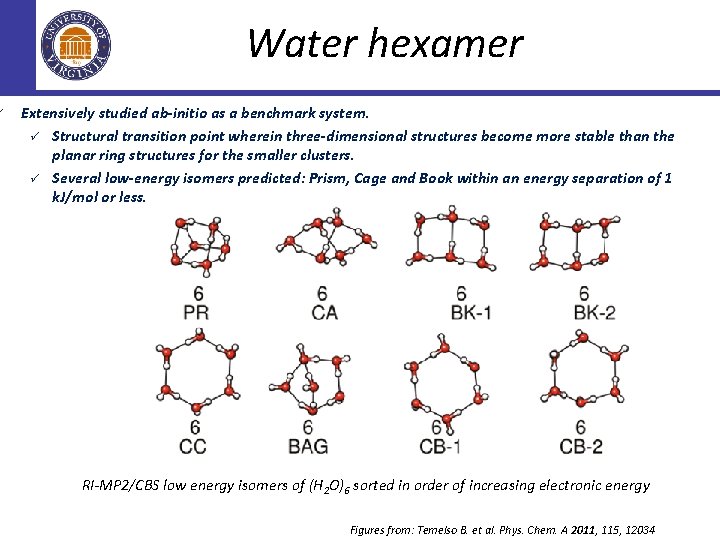
ü Water hexamer Extensively studied ab-initio as a benchmark system. ü Structural transition point wherein three-dimensional structures become more stable than the planar ring structures for the smaller clusters. ü Several low-energy isomers predicted: Prism, Cage and Book within an energy separation of 1 k. J/mol or less. RI-MP 2/CBS low energy isomers of (H 2 O)6 sorted in order of increasing electronic energy Figures from: Temelso B. et al. Phys. Chem. A 2011, 115, 12034

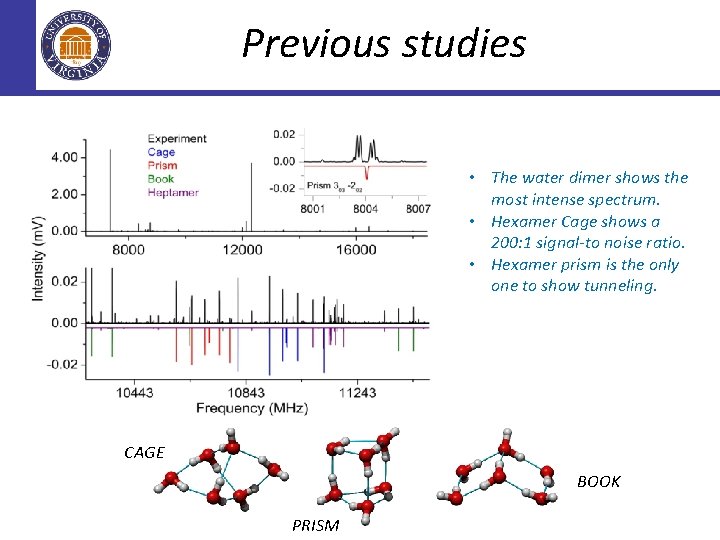
Previous studies • The water dimer shows the most intense spectrum. • Hexamer Cage shows a 200: 1 signal-to noise ratio. • Hexamer prism is the only one to show tunneling. CAGE BOOK PRISM

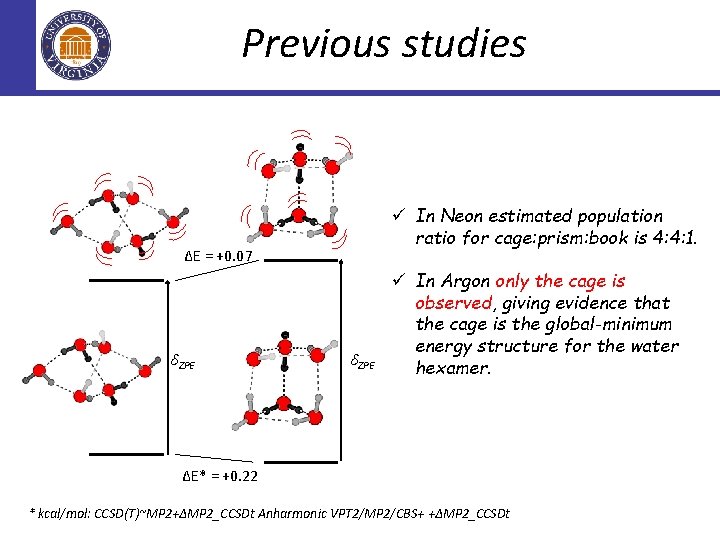
Previous studies ü In Neon estimated population ratio for cage: prism: book is 4: 4: 1. ∆E = +0. 07 δZPE ü In Argon only the cage is observed, giving evidence that the cage is the global-minimum energy structure for the water hexamer. ∆E* = +0. 22 * kcal/mol: CCSD(T)~MP 2+∆MP 2_CCSDt Anharmonic VPT 2/MP 2/CBS+ +∆MP 2_CCSDt

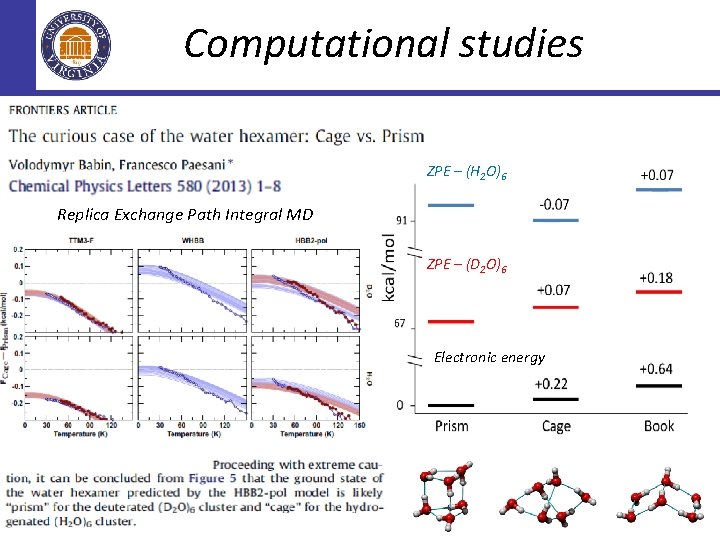
Computational studies ZPE – (H 2 O)6 Replica Exchange Path Integral MD ZPE – (D 2 O)6 Electronic energy

CP-FTMW • CP-FTMW Spectroscopy in the 2 -18 GHz frequency range. • Optimal results found using a gas mixture of neon blown over a reservoir of DI water at a total pressure of 6 atm. • 18 O isotopomer measurements with a reservoir mixture of 1: 6 D 218 O/D 216 O using Ne as carrier gas at a total pressure of 6 atm.

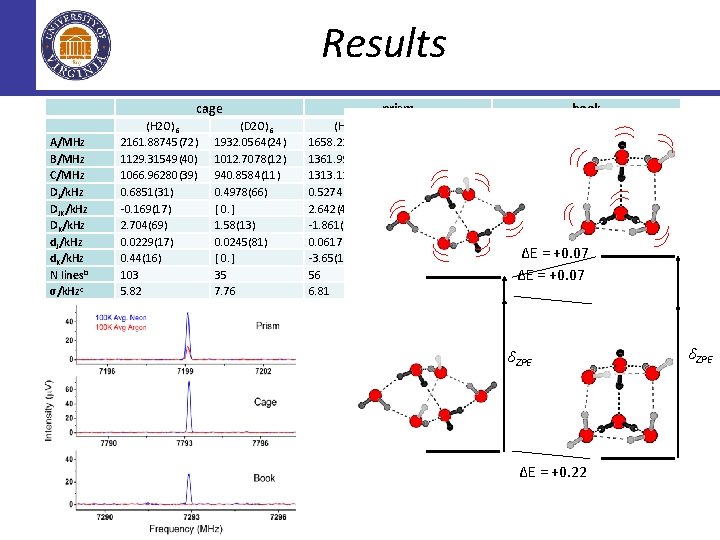
Results cage A/MHz B/MHz C/MHz DJ/k. Hz DJK/k. Hz DK/k. Hz d. J/k. Hz d. K/k. Hz N linesb σ/k. Hzc (H 2 O)6 2161. 88745(72) 1129. 31549(40) 1066. 96280(39) 0. 6851(31) -0. 169(17) 2. 704(69) 0. 0229(17) 0. 44(16) 103 5. 82 (D 2 O)6 1932. 0564(24) 1012. 7078(12) 940. 8584(11) 0. 4978(66) [ 0. ] 1. 58(13) 0. 0245(81) [ 0. ] 35 7. 76 prism (H 2 O)6 1658. 22093(75) 1361. 99984(80) 1313. 12449(74) 0. 5274(92) 2. 642(40) -1. 861(41) 0. 0617(58) -3. 65(15) 56 6. 81 (D 2 O)6 1493. 9052(12) 1218. 3566(12) 1185. 6460(11) 0. 4094(94) 1. 662(58) -0. 961(63) 0. 0511(63) -2. 92(27) 51 8. 23 book (H 2 O)6 (D 2 O)6 1879. 47557(24) 1661. 1986(16) 1063. 98110(17) 940. 6792(28) 775. 06295(13) 690. 23483(77) 0. 9222(12) 0. 452(14) 1. 0201(50) 0. 851(47) 1. 2682(62) [ 0. ] 0. 12496(59) -0. 0121(91) ∆E = +0. 07 2. 1097(58) [ 0. ] 137 ∆E = +0. 07 25 5. 65 9. 33 δZPE ∆E = +0. 22 δZPE

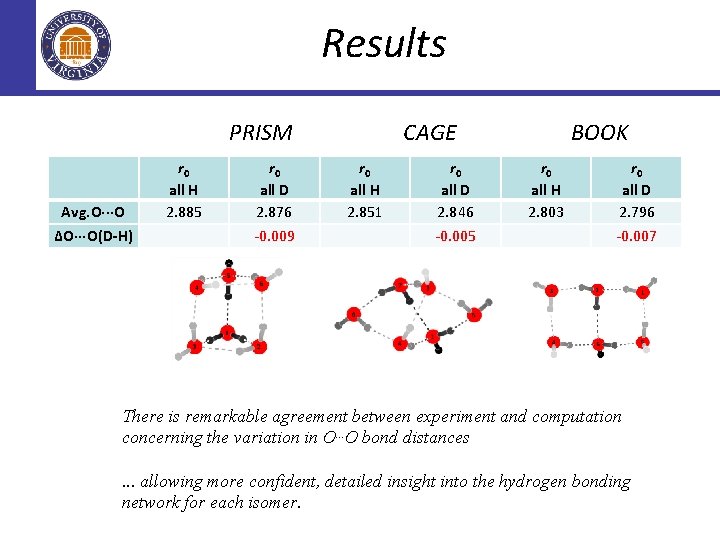
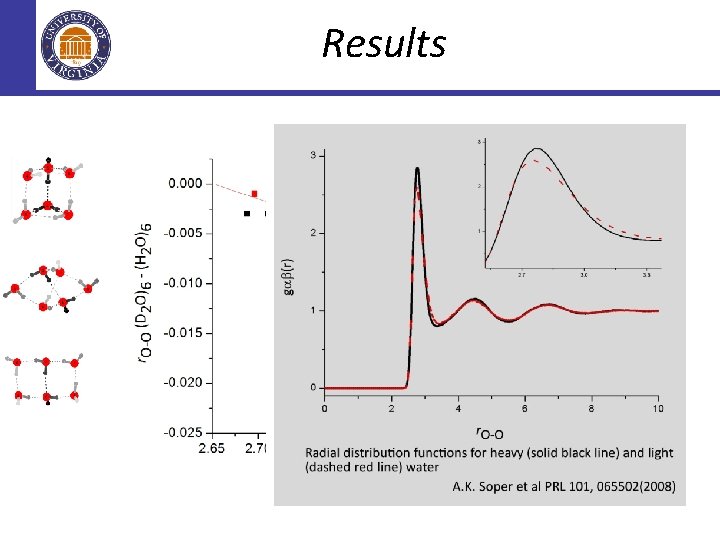
Results PRISM Avg. O···O ∆O···O(D-H) r 0 all H 2. 885 r 0 all D 2. 876 -0. 009 CAGE r 0 all H 2. 851 r 0 all D 2. 846 -0. 005 BOOK r 0 all H 2. 803 r 0 all D 2. 796 -0. 007 There is remarkable agreement between experiment and computation concerning the variation in O. . . O bond distances. . . allowing more confident, detailed insight into the hydrogen bonding network for each isomer.

Results

Acknowledgements Ø Pate Group Ø NSF grants CHE-0960074 Ø MC-IOF 328405

Thank you for your attention Questions / Comments?

This (work) provides a consistent picture of the diversity of hydrogen bonding appearing at the hexamer cluster level. . . which is a small scale prelude to the known diversity in the structure of liquid water.

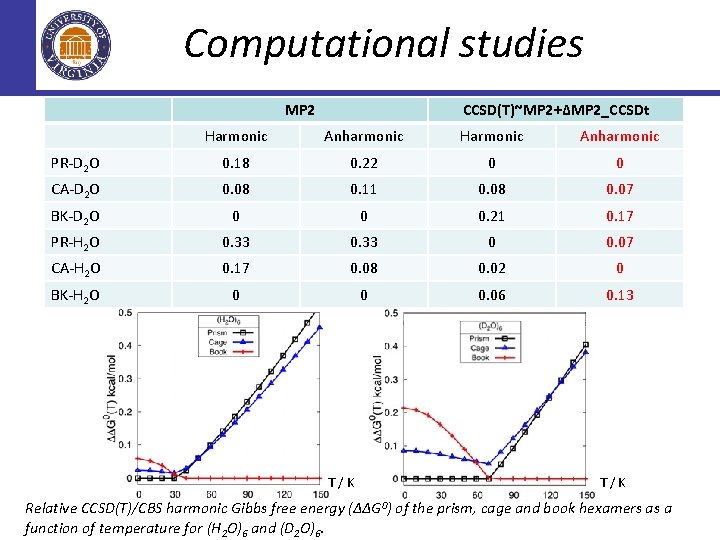
Computational studies MP 2 CCSD(T)~MP 2+∆MP 2_CCSDt Harmonic Anharmonic PR-D 2 O 0. 18 0. 22 0 0 CA-D 2 O 0. 08 0. 11 0. 08 0. 07 BK-D 2 O 0 0 0. 21 0. 17 PR-H 2 O 0. 33 0 0. 07 CA-H 2 O 0. 17 0. 08 0. 02 0 BK-H 2 O 0 0 0. 06 0. 13 T/K Relative CCSD(T)/CBS harmonic Gibbs free energy (∆∆G 0) of the prism, cage and book hexamers as a function of temperature for (H 2 O)6 and (D 2 O)6.

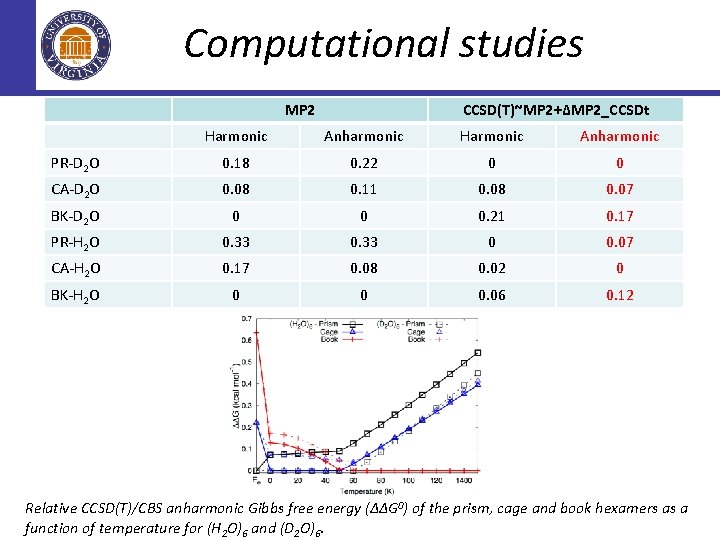
Computational studies MP 2 CCSD(T)~MP 2+∆MP 2_CCSDt Harmonic Anharmonic PR-D 2 O 0. 18 0. 22 0 0 CA-D 2 O 0. 08 0. 11 0. 08 0. 07 BK-D 2 O 0 0 0. 21 0. 17 PR-H 2 O 0. 33 0 0. 07 CA-H 2 O 0. 17 0. 08 0. 02 0 BK-H 2 O 0 0 0. 06 0. 12 Relative CCSD(T)/CBS anharmonic Gibbs free energy (∆∆G 0) of the prism, cage and book hexamers as a function of temperature for (H 2 O)6 and (D 2 O)6.

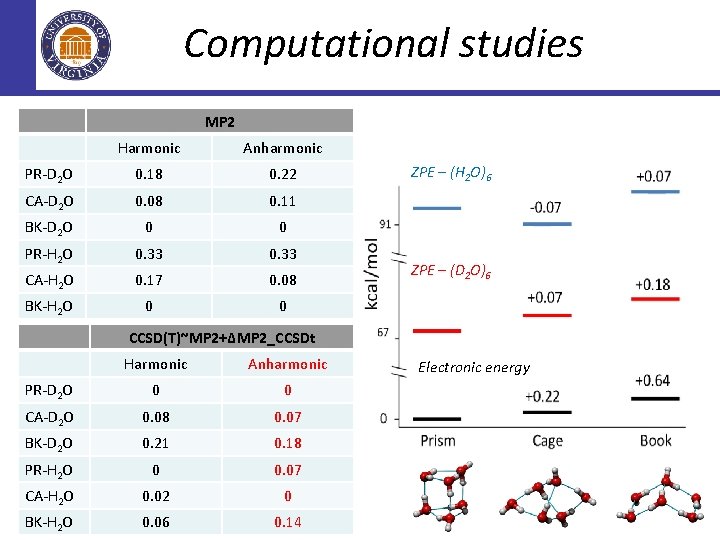
Computational studies MP 2 Harmonic Anharmonic PR-D 2 O 0. 18 0. 22 CA-D 2 O 0. 08 0. 11 BK-D 2 O 0 0 PR-H 2 O 0. 33 CA-H 2 O 0. 17 0. 08 BK-H 2 O 0 0 ZPE – (H 2 O)6 ZPE – (D 2 O)6 CCSD(T)~MP 2+∆MP 2_CCSDt Harmonic Anharmonic PR-D 2 O 0 0 CA-D 2 O 0. 08 0. 07 BK-D 2 O 0. 21 0. 18 PR-H 2 O 0 0. 07 CA-H 2 O 0. 02 0 BK-H 2 O 0. 06 0. 14 Electronic energy
 International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Applications of uv spectroscopy
Applications of uv spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Catalysis lecture notes
Catalysis lecture notes Upcdms
Upcdms International police executive symposium
International police executive symposium Ips perforating
Ips perforating International perforating symposium
International perforating symposium Ips perforating
Ips perforating Ips perforating
Ips perforating International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium Melting and boiling point of oxygen
Melting and boiling point of oxygen Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure For today's meeting
For today's meeting