OSU International Symposium on Molecular Spectroscopy meeting June



- Slides: 11

OSU International Symposium on Molecular Spectroscopy meeting, June 19 -23, in Columbus, Ohio, USA Microwave spectra of 3 -amino-2 -propenenitrile (H 2 N-CH=CH-CN), a molecule of astrochemical interest Thérèse R. Huet, Juan-Ramon Aviles-Moreno, Jean Demaison Laboratoire de Physique des Lasers, Atomes et Molécules UMR 8523 CNRS – Université Lille 1, 59655 Villeneuve d’Ascq Cedex, France Eva Askeland, Harald Møllendal, Einar Uggerud Department of Chemistry, University of Oslo, 0315 Oslo, Norway Jean-Claude Guillemin UMR 6226 CNRS – Sciences Chimiques de Rennes Ecole Nationale Superieure de Chimie de Rennes, 35700 Rennes, France

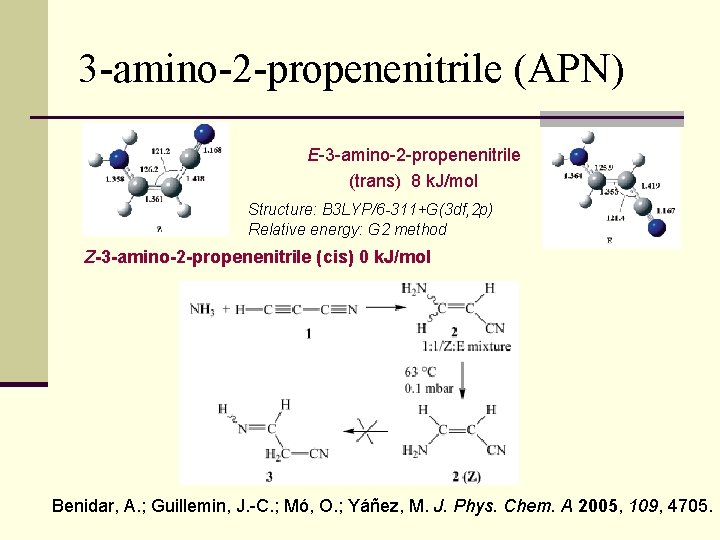
3 -amino-2 -propenenitrile (APN) E-3 -amino-2 -propenenitrile (trans) 8 k. J/mol Structure: B 3 LYP/6 -311+G(3 df, 2 p) Relative energy: G 2 method Z-3 -amino-2 -propenenitrile (cis) 0 k. J/mol Benidar, A. ; Guillemin, J. -C. ; Mó, O. ; Yáñez, M. J. Phys. Chem. A 2005, 109, 4705.

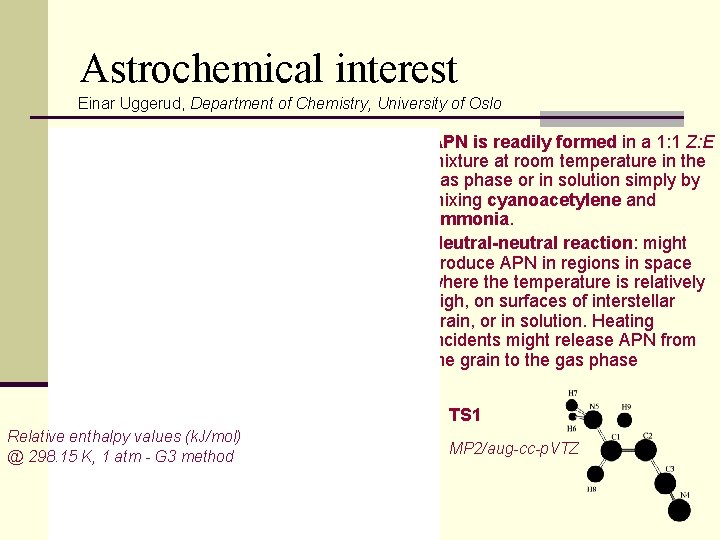
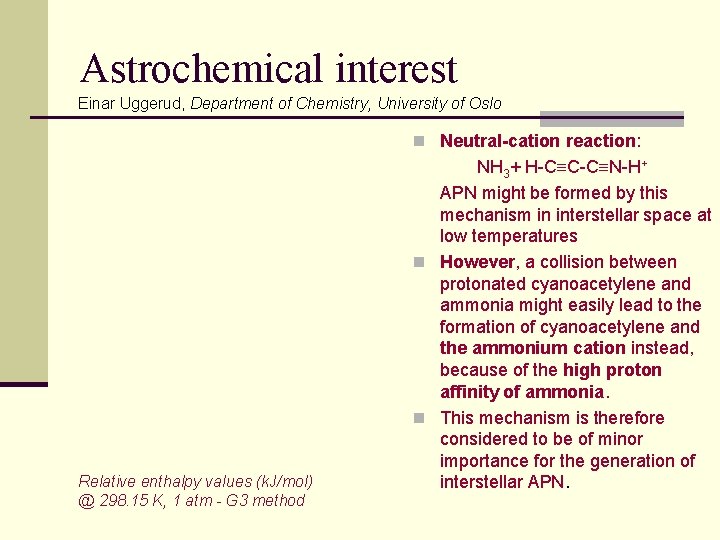
Astrochemical interest Einar Uggerud, Department of Chemistry, University of Oslo n APN is readily formed in a 1: 1 Z: E mixture at room temperature in the gas phase or in solution simply by mixing cyanoacetylene and ammonia. n Neutral-neutral reaction: might produce APN in regions in space where the temperature is relatively high, on surfaces of interstellar grain, or in solution. Heating incidents might release APN from the grain to the gas phase TS 1 Relative enthalpy values (k. J/mol) @ 298. 15 K, 1 atm - G 3 method MP 2/aug-cc-p. VTZ

Astrochemical interest Einar Uggerud, Department of Chemistry, University of Oslo n Neutral-cation reaction: Relative enthalpy values (k. J/mol) @ 298. 15 K, 1 atm - G 3 method NH 3+ H-C C-C N-H+ APN might be formed by this mechanism in interstellar space at low temperatures n However, a collision between protonated cyanoacetylene and ammonia might easily lead to the formation of cyanoacetylene and the ammonium cation instead, because of the high proton affinity of ammonia. n This mechanism is therefore considered to be of minor importance for the generation of interstellar APN.

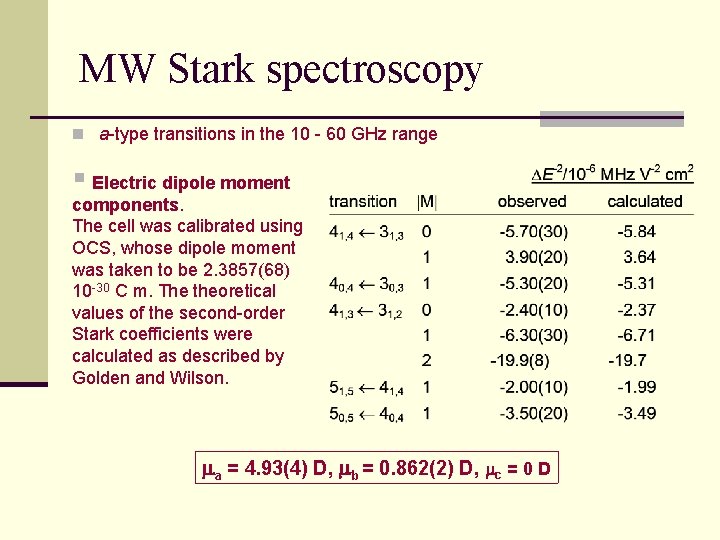
MW Stark spectroscopy n a-type transitions in the 10 - 60 GHz range § Electric dipole moment components. The cell was calibrated using OCS, whose dipole moment was taken to be 2. 3857(68) 10 -30 C m. The theoretical values of the second-order Stark coefficients were calculated as described by Golden and Wilson. ma = 4. 93(4) D, mb = 0. 862(2) D, mc = 0 D

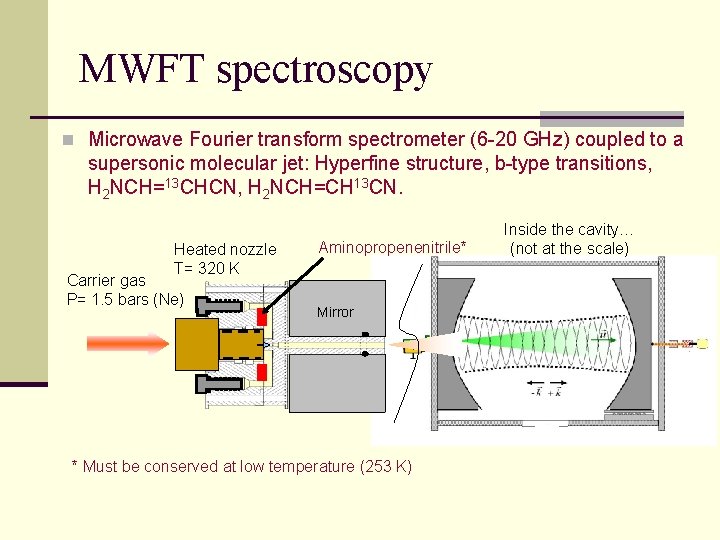
MWFT spectroscopy n Microwave Fourier transform spectrometer (6 -20 GHz) coupled to a supersonic molecular jet: Hyperfine structure, b-type transitions, H 2 NCH=13 CHCN, H 2 NCH=CH 13 CN. Heated nozzle T= 320 K Carrier gas P= 1. 5 bars (Ne) Aminopropenenitrile* Mirror * Must be conserved at low temperature (253 K) Inside the cavity… (not at the scale)

Quadrupolar hyperfine structure The lines are assigned with the I, F quantum numbers, I(N)=1 H 2 N-CH=CH-CN

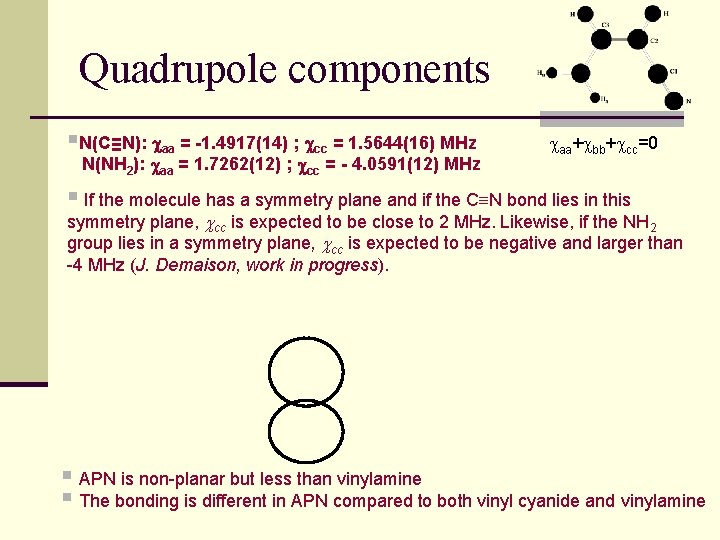
Quadrupole components §N(C≡N): aa = -1. 4917(14) ; cc = 1. 5644(16) MHz N(NH 2): aa = 1. 7262(12) ; cc = - 4. 0591(12) MHz caa+cbb+ccc=0 § If the molecule has a symmetry plane and if the C N bond lies in this symmetry plane, cc is expected to be close to 2 MHz. Likewise, if the NH 2 group lies in a symmetry plane, cc is expected to be negative and larger than -4 MHz (J. Demaison, work in progress). § APN is non-planar but less than vinylamine § The bonding is different in APN compared to both vinyl cyanide and vinylamine

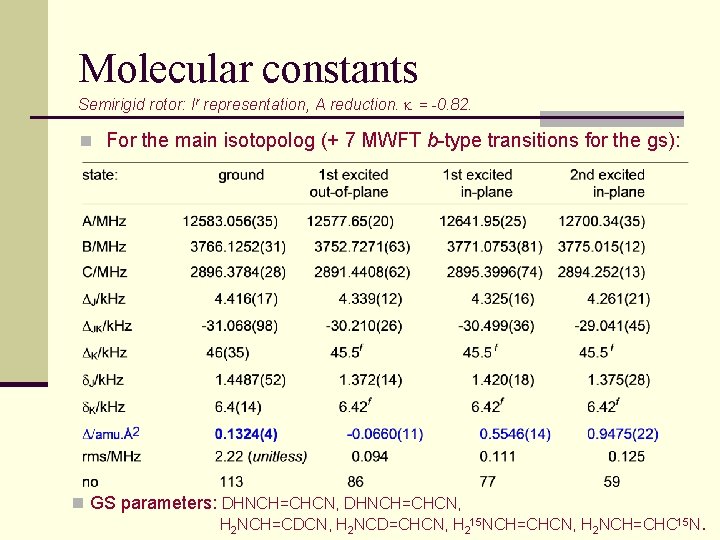
Molecular constants Semirigid rotor: Ir representation, A reduction. k = -0. 82. n For the main isotopolog (+ 7 MWFT b-type transitions for the gs): n GS parameters: DHNCH=CHCN, H 2 NCH=CDCN, H 2 NCD=CHCN, H 215 NCH=CHCN, H 2 NCH=CHC 15 N.

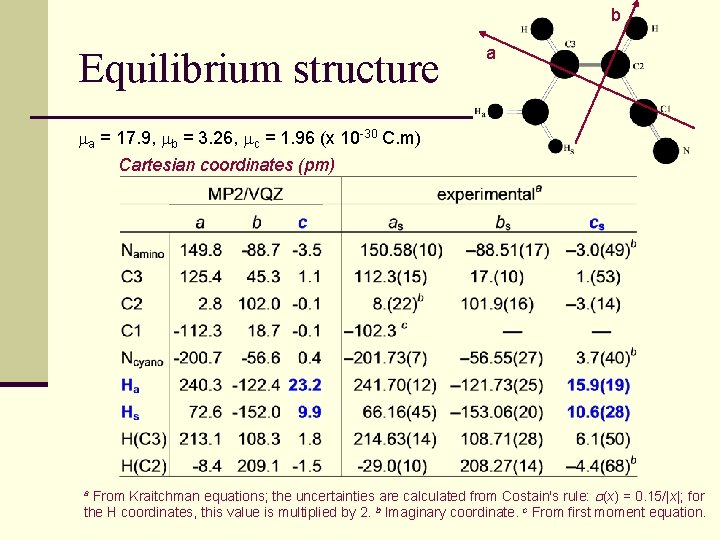
b Equilibrium structure a ma = 17. 9, mb = 3. 26, mc = 1. 96 (x 10 -30 C. m) Cartesian coordinates (pm) From Kraitchman equations; the uncertainties are calculated from Costain's rule: (x) = 0. 15/|x|; for the H coordinates, this value is multiplied by 2. b Imaginary coordinate. c From first moment equation. a

Acknowledgment n The Institut du Développement des Ressources en Informatique Scientifique (contract IDRIS 51715, France) n The Programme National de Physico-Chimie du Milieu Interstellaire (PCMI, France) n The French-Norwegian Aurora Program n The Research Council of Norway (Program for Supercomputing)
 International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Applications of uv visible spectroscopy
Applications of uv visible spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Catalysis lecture notes
Catalysis lecture notes Jpl molecular spectroscopy
Jpl molecular spectroscopy International police executive symposium
International police executive symposium Ips perforating
Ips perforating International perforating symposium
International perforating symposium Chara screenshot
Chara screenshot Ips perforating
Ips perforating International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium