OSU International Symposium on Molecular Spectroscopy meeting June
- Slides: 12

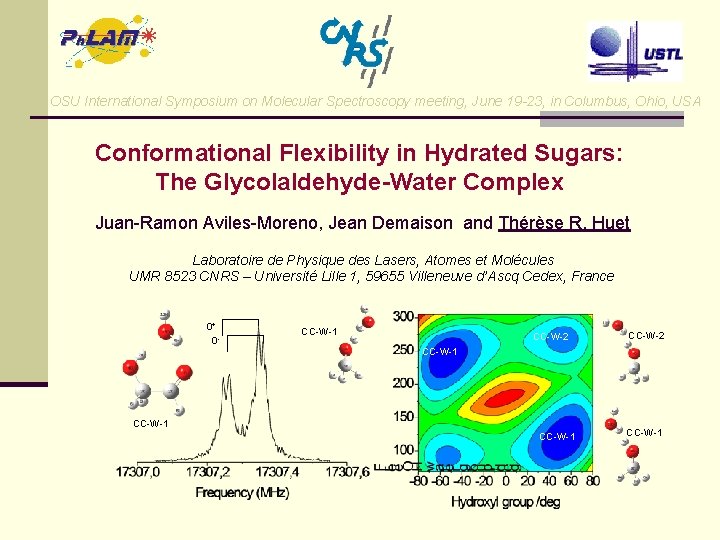
OSU International Symposium on Molecular Spectroscopy meeting, June 19 -23, in Columbus, Ohio, USA Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex Juan-Ramon Aviles-Moreno, Jean Demaison and Thérèse R. Huet Laboratoire de Physique des Lasers, Atomes et Molécules UMR 8523 CNRS – Université Lille 1, 59655 Villeneuve d’Ascq Cedex, France 0+ 0 - CC-W-1 CC-W-2 CC-W-1

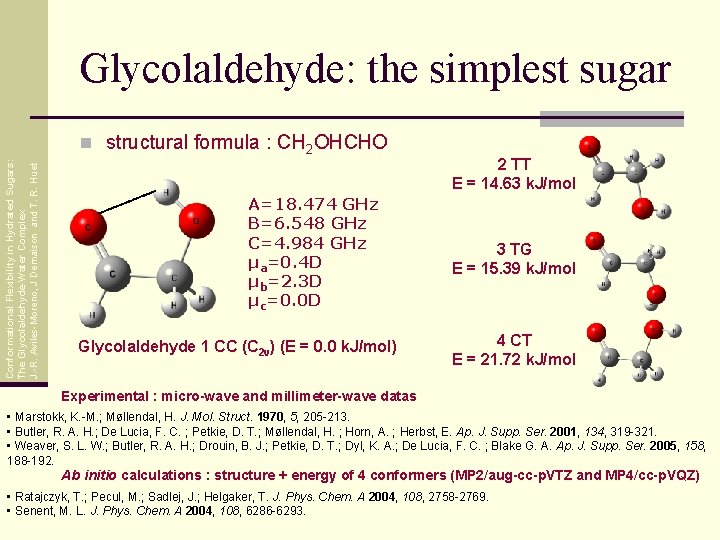
Glycolaldehyde: the simplest sugar Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet n structural formula : CH 2 OHCHO A=18. 474 GHz B=6. 548 GHz C=4. 984 GHz μa=0. 4 D μb=2. 3 D μc=0. 0 D Glycolaldehyde 1 CC (C 2 v) (E = 0. 0 k. J/mol) 2 TT E = 14. 63 k. J/mol 3 TG E = 15. 39 k. J/mol 4 CT E = 21. 72 k. J/mol Experimental : micro-wave and millimeter-wave datas • Marstokk, K. -M. ; Møllendal, H. J. Mol. Struct. 1970, 5, 205 -213. • Butler, R. A. H. ; De Lucia, F. C. ; Petkie, D. T. ; Møllendal, H. ; Horn, A. ; Herbst, E. Ap. J. Supp. Ser. 2001, 134, 319 -321. • Weaver, S. L. W. ; Butler, R. A. H. ; Drouin, B. J. ; Petkie, D. T. ; Dyl, K. A. ; De Lucia, F. C. ; Blake G. A. Ap. J. Supp. Ser. 2005, 158, 188 -192. Ab initio calculations : structure + energy of 4 conformers (MP 2/aug-cc-p. VTZ and MP 4/cc-p. VQZ) • Ratajczyk, T. ; Pecul, M. ; Sadlej, J. ; Helgaker, T. J. Phys. Chem. A 2004, 108, 2758 -2769. • Senent, M. L. J. Phys. Chem. A 2004, 108, 6286 -6293.

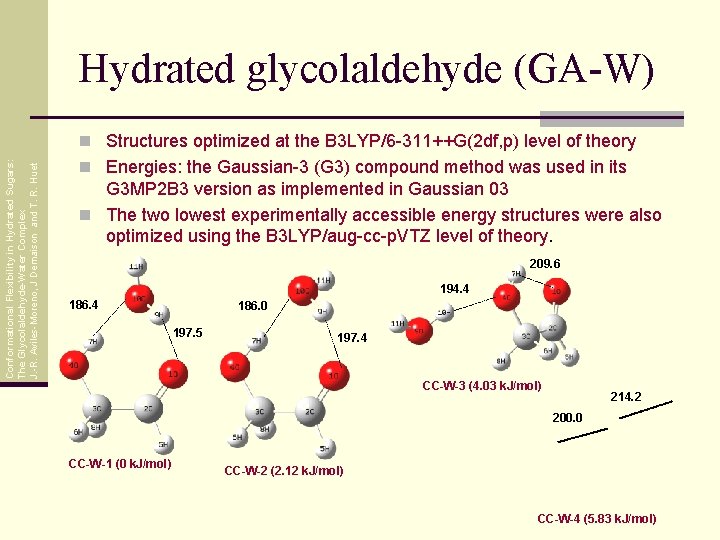
Hydrated glycolaldehyde (GA-W) Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet n Structures optimized at the B 3 LYP/6 -311++G(2 df, p) level of theory n Energies: the Gaussian-3 (G 3) compound method was used in its G 3 MP 2 B 3 version as implemented in Gaussian 03 n The two lowest experimentally accessible energy structures were also optimized using the B 3 LYP/aug-cc-p. VTZ level of theory. 209. 6 194. 4 186. 0 197. 5 197. 4 CC-W-3 (4. 03 k. J/mol) 214. 2 200. 0 CC-W-1 (0 k. J/mol) CC-W-2 (2. 12 k. J/mol) CC-W-4 (5. 83 k. J/mol)

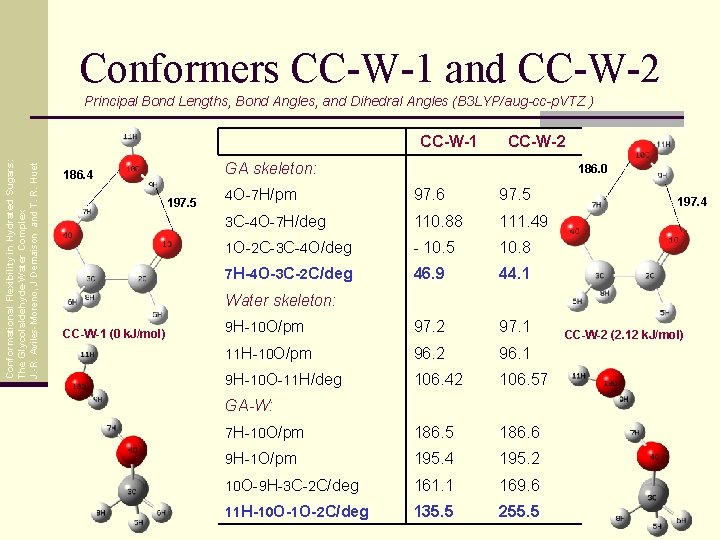
Conformers CC-W-1 and CC-W-2 Principal Bond Lengths, Bond Angles, and Dihedral Angles (B 3 LYP/aug-cc-p. VTZ ) Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet CC-W-1 CC-W-2 GA skeleton: 186. 4 197. 5 186. 0 4 O-7 H/pm 97. 6 97. 5 3 C-4 O-7 H/deg 110. 88 111. 49 1 O-2 C-3 C-4 O/deg - 10. 5 10. 8 7 H-4 O-3 C-2 C/deg 46. 9 44. 1 9 H-10 O/pm 97. 2 97. 1 11 H-10 O/pm 96. 2 96. 1 9 H-10 O-11 H/deg 106. 42 106. 57 7 H-10 O/pm 186. 5 186. 6 9 H-1 O/pm 195. 4 195. 2 10 O-9 H-3 C-2 C/deg 161. 1 169. 6 11 H-10 O-1 O-2 C/deg 135. 5 255. 5 197. 4 Water skeleton: CC-W-1 (0 k. J/mol) GA-W: CC-W-2 (2. 12 k. J/mol)

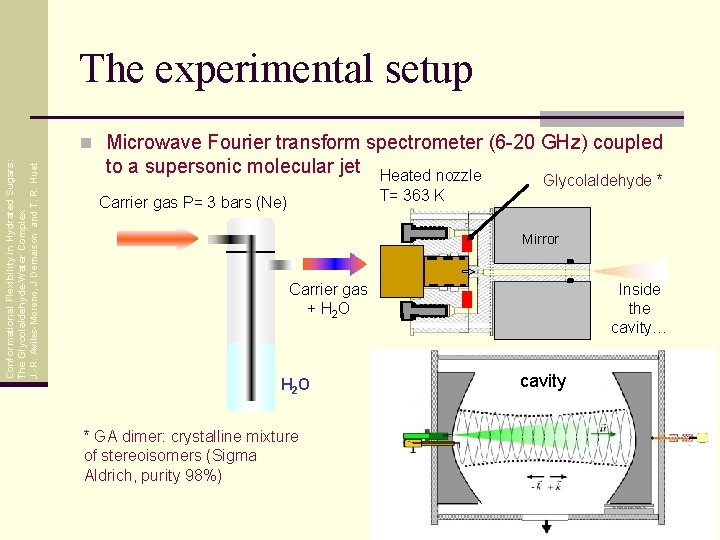
The experimental setup Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet n Microwave Fourier transform spectrometer (6 -20 GHz) coupled to a supersonic molecular jet Carrier gas P= 3 bars (Ne) Heated nozzle T= 363 K Glycolaldehyde * Mirror Carrier gas + H 2 O H 2 O * GA dimer: crystalline mixture of stereoisomers (Sigma Aldrich, purity 98%) Inside the cavity… cavity

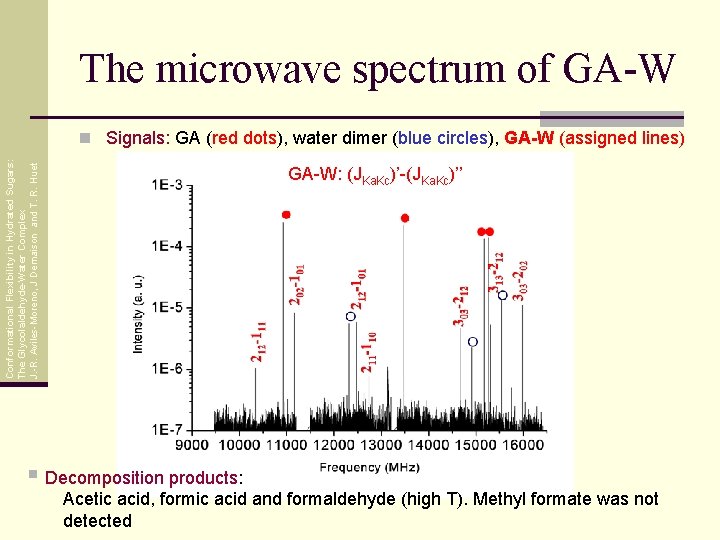
The microwave spectrum of GA-W Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet n Signals: GA (red dots), water dimer (blue circles), GA-W (assigned lines) GA-W: (JKa. Kc)’-(JKa. Kc)’’ § Decomposition products: Acetic acid, formic acid and formaldehyde (high T). Methyl formate was not detected

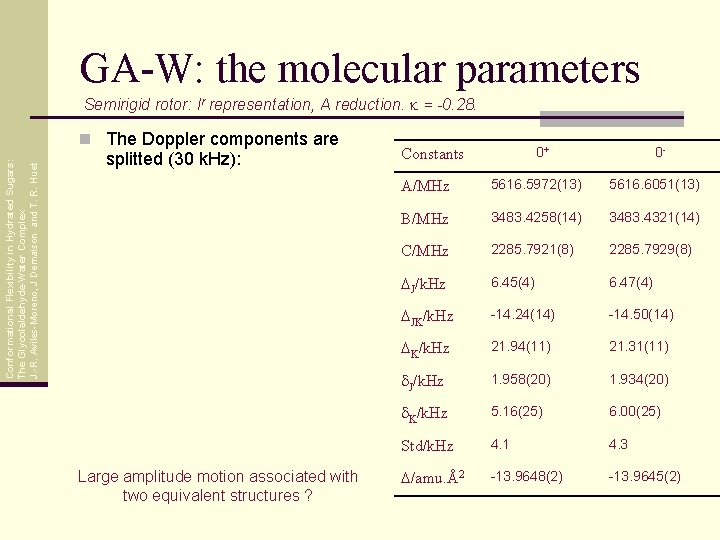
GA-W: the molecular parameters Semirigid rotor: Ir representation, A reduction. k = -0. 28. Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet n The Doppler components are splitted (30 k. Hz): Large amplitude motion associated with two equivalent structures ? 0+ Constants 0 - A/MHz 5616. 5972(13) 5616. 6051(13) B/MHz 3483. 4258(14) 3483. 4321(14) C/MHz 2285. 7921(8) 2285. 7929(8) DJ/k. Hz 6. 45(4) 6. 47(4) DJK/k. Hz -14. 24(14) -14. 50(14) DK/k. Hz 21. 94(11) 21. 31(11) d. J/k. Hz 1. 958(20) 1. 934(20) d. K/k. Hz 5. 16(25) 6. 00(25) Std/k. Hz 4. 1 4. 3 D/amu. Å2 -13. 9648(2) -13. 9645(2)

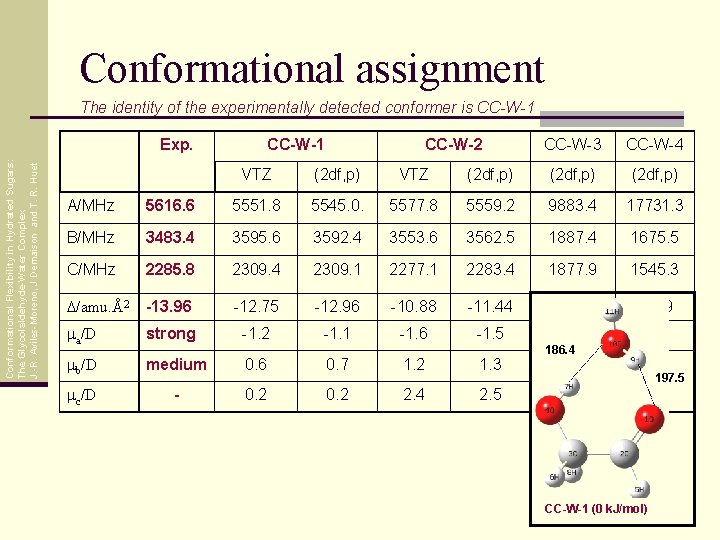
Conformational assignment The identity of the experimentally detected conformer is CC-W-1 Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet Exp. CC-W-1 CC-W-2 CC-W-3 CC-W-4 VTZ (2 df, p) A/MHz 5616. 6 5551. 8 5545. 0. 5577. 8 5559. 2 9883. 4 17731. 3 B/MHz 3483. 4 3595. 6 3592. 4 3553. 6 3562. 5 1887. 4 1675. 5 C/MHz 2285. 8 2309. 4 2309. 1 2277. 1 2283. 4 1877. 9 1545. 3 D/amu. Å2 -13. 96 -12. 75 -12. 96 -10. 88 -11. 44 -49. 78 -3. 09 ma/D strong -1. 2 -1. 1 -1. 6 -1. 5 -0. 5 0. 1 mb/D medium 0. 6 0. 7 1. 2 1. 3 mc/D - 0. 2 2. 4 2. 5 186. 4 1. 5 0. 6 1. 4 0. 0 197. 5 CC-W-1 (0 k. J/mol)

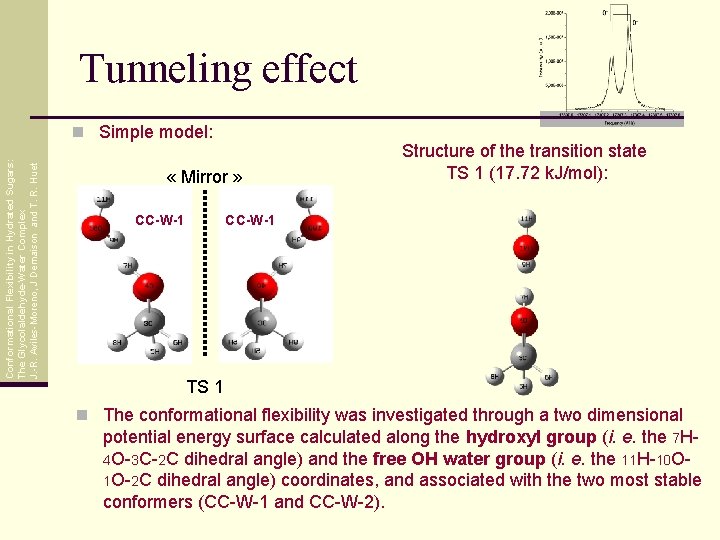
Tunneling effect Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet n Simple model: « Mirror » CC-W-1 Structure of the transition state TS 1 (17. 72 k. J/mol): CC-W-1 TS 1 n The conformational flexibility was investigated through a two dimensional potential energy surface calculated along the hydroxyl group (i. e. the 7 H 4 O-3 C-2 C dihedral angle) and the free OH water group (i. e. the 11 H-10 O 1 O-2 C dihedral angle) coordinates, and associated with the two most stable conformers (CC-W-1 and CC-W-2).

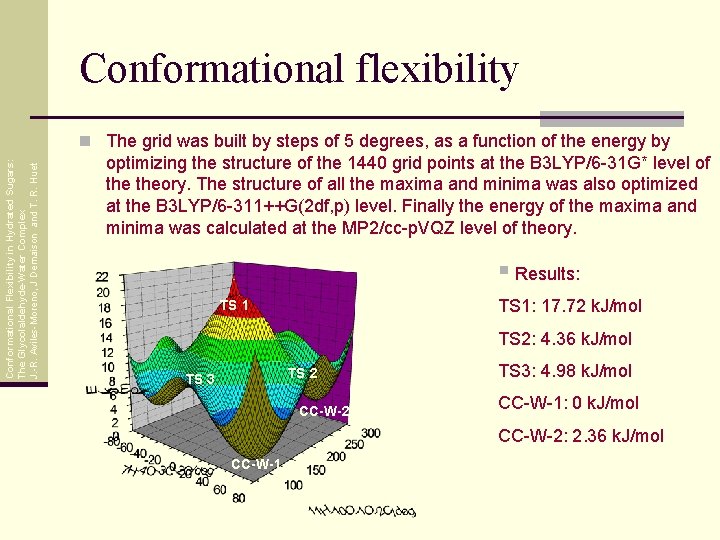
Conformational flexibility Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet n The grid was built by steps of 5 degrees, as a function of the energy by optimizing the structure of the 1440 grid points at the B 3 LYP/6 -31 G* level of theory. The structure of all the maxima and minima was also optimized at the B 3 LYP/6 -311++G(2 df, p) level. Finally the energy of the maxima and minima was calculated at the MP 2/cc-p. VQZ level of theory. § Results: TS 1: 17. 72 k. J/mol TS 1 TS 2: 4. 36 k. J/mol TS 2 TS 3 CC-W-2 TS 3: 4. 98 k. J/mol CC-W-1: 0 k. J/mol CC-W-2: 2. 36 k. J/mol CC-W-1

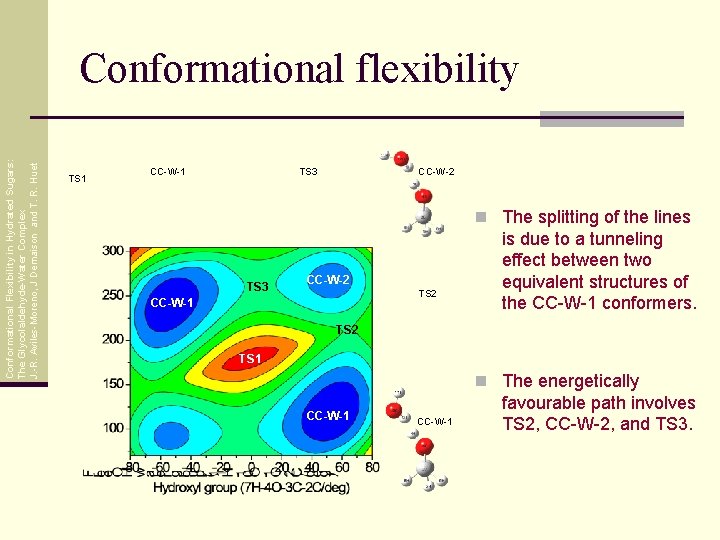
Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet Conformational flexibility TS 1 CC-W-1 TS 3 CC-W-2 n The splitting of the lines TS 3 CC-W-2 TS 2 CC-W-1 is due to a tunneling effect between two equivalent structures of the CC-W-1 conformers. TS 2 TS 1 n The energetically CC-W-1 favourable path involves TS 2, CC-W-2, and TS 3.

Acknowledgment Conformational Flexibility in Hydrated Sugars: The Glycolaldehyde-Water Complex J. -R. Aviles-Moreno, J Demaison and T. R. Huet n The Institut du Développement des Ressources en Informatique Scientifique (contract IDRIS 51715, France) n The Programme National de Physico-Chimie du Milieu Interstellaire (PCMI, France) Manuscript submitted to the J. Am. Chem. Soc. 0+ 0 - CC-W-1 CC-W-2 CC-W-1
 International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Applications of uv spectroscopy
Applications of uv spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Catalysis lecture notes
Catalysis lecture notes Upcdms
Upcdms International police executive symposium
International police executive symposium Ips perforating
Ips perforating International perforating symposium
International perforating symposium Ips perforating
Ips perforating International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium Covalently bonded substances
Covalently bonded substances